Silver nanoparticles supported on silica-coated ferrite as magnetic and reusable catalysts for oxidant-free alcohol dehydrogenation
Ahmad Bayat,
Mehdi Shakourian-Fard,
Nona Ehyaei and
Mohammad Mahmoodi Hashemi*
Department of Chemistry, Sharif University of Technology, PO Box 11465-9516, Tehran, Iran. E-mail: mhashemi@sharif.edu
First published on 20th January 2015
Abstract
Silver (0) nanoparticles supported on silica-coated ferrite were synthesized to be used as an efficient and recyclable heterogeneous catalyst for oxidant-free dehydrogenation of alcohols to the corresponding carbonyl compounds. The catalyst can be easily recovered and reused for 8 reaction cycles without considerable loss of activity. The facile recovery of the catalyst is carried out by applying an external magnetic device. The catalyst was fully characterized by the techniques of TEM, SEM, XRD, EDS, ICP-AES, and VSM.
Introduction
The dehydrogenation of alcohols to the corresponding aldehydes and ketones is one of the most important and fascinating reactions in organic chemistry because carbonyl compounds serve as important precursors and intermediates for many drugs, vitamins and fragrances.1–3 Traditional oxidation of alcohols is performed by non-catalytic methods. A stoichiometry amount of oxidants such as dichromate is required to affect this oxidation process and a large amount of toxic waste would be generated.4,5Recently, much effort has been made to develop heterogeneous and homogeneous catalysts to solve these problems. So far, some interesting transition metal catalysts including Au,6 Pd,7 Pt,8 Co,9 Ru,10 Mn11 and metal-free catalysts such as TEMPO12 and mesoporous carbon nitride (mpg-C3N4) polymer13 in the presence of a suitable oxidant such as H2O214 and molecular oxygen15 have been reported for the catalytic dehydrogenation of alcohols. However, the oxidation of alcohol in the presence of oxygen molecules has some drawbacks, for example oxygen may cause overoxidation and explosion or combustion of the organic solvents or alcohol reactants. One of the advantages of the oxidant-free dehydrogenation of alcohols is tolerance toward alcohols having O2-sensitive functional groups.16–18 Therefore, from the viewpoint of safety of the reaction, oxidant-free and acceptor-free catalytic dehydrogenation of alcohols is ideal.
Up to now, various homogeneous catalytic systems for the oxidant-free dehydrogenation of alcohols to the corresponding carbonyl compounds using ruthenium,19 iridium,20 and other transition metal catalysts have been reported. However, these systems suffer variously from drawbacks such as difficulties in catalyst synthesis, manipulation and reusability and requirement of acid or base additives.
One way to solve this drawback is to immobilize catalytic system onto a large surface area solid carrier and make a heterogeneous catalyst system.
Silver-based heterogeneous catalysts have been frequently used for oxidation of alcohols. It was shown that Ag-containing catalysts supported on basic and acidic oxides are very active in the oxidant-free dehydrogenation of alcohols.21–23 For example, Ivanova et al. used Ag/SiO2 for the oxidant-free dehydrogenation of ethanol.18 Also, Mitsudome et al. studied application of Ag/hydrotalcite for none-oxidant dehydrogenation of secondary alcohols.24 Most of these heterogeneous systems that used for oxidant-free dehydrogenation of alcohols require a filtration or centrifugation step or a tedious workup of the final reaction mixture to recover the catalyst. One of the best ways for recovering the catalyst is using catalyst system supported on magnetic nanoparticles. Moreover, the use of magnetic nanoparticles enable their separation process from reaction mixture by an external magnet and reuse of the catalyst itself.25–27 Therefore, separation by external magnet offering a promising option that can meet the requirements of high accessibility with improved reusability without need to filtration or centrifugation step.
According to the best of our knowledge, there is no published work about supported silver nanoparticles on magnetic nanoparticles for oxidant-free dehydrogenation of alcohols. We report our results about the highly effective acceptor-free and oxidant-free dehydrogenation of alcohols using heterogeneous silver nanoparticles supported on silica-coated ferrite (Fe3O4@SiO2-Ag) composite as recoverable heterogeneous catalyst. This catalyst system showed exceptionally high activity for various types of alcohols.
Characterization
Reagents and analyses
All chemicals and solvents were purchased from Sigma-Aldrich and used without further purification. Ag content of the catalyst sample was determined by inductively coupled plasma optical emission spectroscopy (Optima 800 ICP-OES spectrometer). X-ray diffraction (XRD) patterns of prepared catalyst were recorded with a APD 2000 PRO 2010, using Cu Kα radiation (50 kV, 150 mA) in the range 2θ = 10–90°. The scanning electron microscopy (SEM) was done with KYKY-EM3200 with maximum acceleration voltage of the primary electron between 20 and 25 kV. An energy dispersive detector (EDS) coupled to the microscope was used to indentify chemical elements of the prepared catalyst. The transmission electron microscopy (TEM) image was obtained with Philips CM30 TEM. The room-temperature magnetization in the applied magnetic field was performed by a homemade vibrating sample magnetometer (Meghnatis Daghigh Kavir Company, Iran) from −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to +10
000 to +10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe.
000 Oe.
Preparation of magnetic nanoparticles (Fe3O4)
The Fe3O4 magnetic nanoparticles were prepared as reported in literature.28 Briefly, FeCl3·6H2O (4.8 g, 0.018 mol) and FeCl2·4H2O (1.8 g, 0.0089 mol) were added to 100 mL deionized water and vigorously stirred (700 rpm) under N2 atmosphere until the salts dissolved completely. Then, 10 mL of 25% NH4OH was added dropwise over 20 min into the reaction mixture at room temperature, which the black precipitate of magnetic nanoparticles (MNPs) was formed immediately. After continuously stirring by mechanical stirrer for 1 h, the precipitate was separated by an external magnet, washed with the double distilled water (five times), and then vacuum-dried at 50 °C for overnight.Fe3O4 magnetic nanoparticles (MNPs) coated by silica (Fe3O4@SiO2)
The prepared MNPs (1 g) were homogeneously dispersed in the mixture of ethanol (80 mL) and deionized water (20 mL) and then pH of solution was adjusted to 10 by adding aqueous ammonia. Tetraethoxysilane (TEOS, 0.42 mmol) was added to the solution and stirred at 50 °C for 6 h to obtain Fe3O4@SiO2 MNPs. After washing with ethanol and water for several times, Fe3O4@SiO2 MNPs were dried at 60 °C for overnight.Deposition of silver nanoparticles on Fe3O4@SiO2 surface
Silver was deposited on the Fe3O4@SiO2 surface as follows: a AgNO3 (0.05 g) solution was added into the well dispersed Fe3O4@SiO2 (1 g) in deionized water, then 0.1 mL of aqueous ammonia (25%, w/w) was added which Ag(NH3)2+ complex was formed. The pH suspension was adjusted to 12.0 with NaOH solution and the mixture was stirred for 30 min. Then, an aqueous solution of NaBH4 (0.1 M) was added dropwise to mixture to reduce Ag(NH3)2+ complex to Ag nanoparticles on the Fe3O4@SiO2 surface. Finally, the mixture was magnetically separated, washed several times with deionized water, and then dried at 50 °C for overnight to afford the Fe3O4@SiO2-Ag (Scheme 1).General procedure for oxidant-free alcohols dehydrogenation
Initially, Fe3O4@SiO2-Ag (15 mg) was added into 3 mL of toluene and sonicated for 5 min. 1 mmol of alcohol was then added to the mixture in a reaction vessel fitted with a condenser, and the system was placed under N2. The resulting mixture was vigorously stirred under reflux conditions. The reaction progress was monitored by TLC (n-hexane/ethylacetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and gas chromatography (GC). After the completion of reaction, the catalyst was separated by applying an external magnet. The residue was evaporated in vacuum, and the crude product was purified by column chromatography on silica gel with ethyl acetate/hexane (1
1) and gas chromatography (GC). After the completion of reaction, the catalyst was separated by applying an external magnet. The residue was evaporated in vacuum, and the crude product was purified by column chromatography on silica gel with ethyl acetate/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to afford the desired product. The separated catalyst was washed with EtOH and used directly for a subsequent round of reaction without further purification. All reaction products listed in Table 2 are known in literature and identified by 1HNMR spectra, IR spectra and melting point as compared with authentic samples.
9) to afford the desired product. The separated catalyst was washed with EtOH and used directly for a subsequent round of reaction without further purification. All reaction products listed in Table 2 are known in literature and identified by 1HNMR spectra, IR spectra and melting point as compared with authentic samples.
| Entry | Catalyst | Catalyst mg (mol%) | Con.b (%) | Sel.b (%) |
|---|---|---|---|---|
| a Reaction conditions: alcohol (1 mmol), toluene (3 mL), under reflux for 24 h.b Determined by GC. | ||||
| 1 | MNP | 5 | Trace | 100 |
| 2 | AgNO3 | 17 (10) | NR | — |
| 3 | Fe3O4@SiO2-Ag | 3.75 (0.05) | 80 | 98 |
| 4 | Fe3O4@SiO2-Ag | 1.5 (0.10) | 86 | 99 |
| 5 | Fe3O4@SiO2-Ag | 11.25 (0.15) | 90 | 98 |
| 6 | Fe3O4@SiO2-Ag | 15 (0.20) | 98 | 99 |
| 7 | Fe3O4@SiO2-Ag | 30 (0.40) | 97 | 98 |
| 8 | Fe3O4@SiO2-Ag | 37.5 (0.50) | 98 | 98 |
| Entry | Substrate | Product | Con.b (%) | Sel.b (%) |
|---|---|---|---|---|
| a Reaction conditions: substrate (1 mmol), Fe3O4@SiO2-Ag 15 mg (0.20 mol% Ag, 2 × 10−3 mmol Ag), toluene (3 mL), reflux, N2 atmosphere, time = 24 h.b Determined by GC.c Fe3O4@SiO2-Ag 30 mg (0.40 mol% Ag).d Time = 48 h. | ||||
| 1 |  |
 |
98 | 98 |
| 2 | 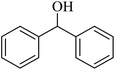 |
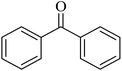 |
>99 | >99 |
| 3 |  |
 |
98 | 97 |
| 4 |  |
 |
>99 | 99 |
| 5 |  |
 |
>99 | 99 |
| 6 |  |
 |
>99 | 99 |
| 7 | 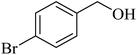 |
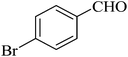 |
98 | 99 |
| 8 |  |
 |
96 | >99 |
| 9 | 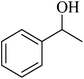 |
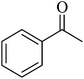 |
98 | 99 |
| 10c,d | 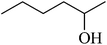 |
 |
68 | 97 |
| 11c,d |  |
 |
55 | 96 |
| 12c,d |  |
 |
94 | 99 |
| 13c,d |  |
 |
91 | 96 |
| 14 |  |
 |
99 | 98 |
| 15c,d |  |
 |
71 | 97 |
| 16 |  |
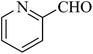 |
>99 | 99 |
| 17 |  |
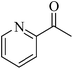 |
98 | 98 |
Results and discussion
Preparation and characterization of Fe3O4@SiO2-Ag
The first step, the bare Fe3O4 was prepared by using a Fe2+/Fe3+ mixture via the conventional coprecipitation method (Scheme 1). The Fe3O4 core trends to be oxidized or dissolved in acidic conditions during the treatment procedure. Thus, the SiO2 was used to protect the magnetic core material against agglomeration and oxidation, providing high surface area to stabilize the silver nanoparticles. Silanol groups in Fe3O4@SiO2 can be deprotonated in alkaline conditions and form nucleophilic parts (Si–O−) which can react with Ag+. It was confirmed that pKa of silanol groups is 7.0 and then the system pH must be at least 9.0 for deprotonate of Si–OH groups.29,30 After deprotonation of silanol groups, Ag+ ions were impregnated on Fe3O4@SiO2 through electrostatic interaction with Si–O− from the aqueous solution of silver(I) nitrate and then reduced by NaBH4 at room temperature. The catalyst was characterized using various methods.Fig. 1 shows the XRD pattern of Fe3O4@SiO2-Ag catalyst with characteristic peaks and relative intensity which matches completely with Fe3O4 sample (Fig. 1a). Diffraction peaks at 2θ = 30.8, 35.4, 43.6, 53.5, 57.7, and 62.4 correspond to (220), (311), (400), (422), (551), and (440) diffraction planes of Fe3O4 MNPs which are in good agreement with the standard XRD data for the cubic Fe3O4 phase of inverse spinel crystal structure (JCPDS Card numbers 89-43191, 19-0629, 79-0419).31
The comparison of XRD pattern of Fe3O4@SiO2 (Fig. 1a) and Fe3O4@SiO2-Ag (Fig. 1b) indicates that these main peaks did not change after the deposition of Ag nanoparticles, indicating retention of the crystalline structure during the deposition. The signals pertaining to silver metal were not detected in XRD of Fe3O4@SiO2-Ag catalyst which indicate that the Ag species are highly dispersed on ferrites.
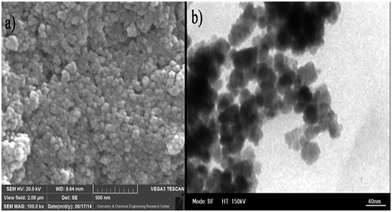
A representative TEM image of Fe3O4@SiO2-Ag catalyst is shown in Fig. 2b, it confirms that most of the particles are in quasi-spherical in shape with an average diameter of approximately 20–30 nm. SEM image (Fig. 2a) of Fe3O4@SiO2-Ag catalyst shows that catalyst is quite uniform.
The elemental analysis maps for Fe, Si, and Ag nanoparticles were obtained by SEM-EDS analysis and summarized in Fig. 3. Fig. 3d shows that Ag nanoparticles as an active component in the catalyst are dispersed completely and no aggregation is seen for them through the catalyst.
In the EDS profile (Fig. 3e) of the Fe3O4@SiO2-Ag, the peaks of Si, Fe, Ag and O are obviously observed, and no other impurities detected. Silver content of Fe3O4@SiO2-Ag catalyst was found to be 1.48% wt by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis.
The magnetic properties of the Fe3O4@SiO2, and Fe3O4@SiO2-Ag catalysts were investigated by a vibrating sample magnetometer at room temperature. As seen in Fig. 4, the magnetization curves of the prepared materials exhibit no hysteresis loop which demonstrates its supermagnetic characteristics. The deposition of silver on Fe3O4@SiO2 causes low decreasing of saturation magnetization from 70 emu g−1 (a) to exceeding 59 emu g−1 (b) for Fe3O4@SiO2-Ag. Therefore, the strong magnetic properties of the prepared catalyst were revealed by complete and easy attraction using an external magnet which minimizes the loss of catalyst during the separation stage. The above results suggested strongly that the Fe3O4@SiO2-Ag catalyst was prepared by a simple and practical method.
 | ||
| Fig. 4 Magnetization curves of Fe3O4 (a), Fe3O4@SiO2 (b), and Fe3O4@SiO2-Ag catalyst (c). The inset is a photograph of Fe3O4@SiO2-Ag catalyst under an external magnetic field. | ||
Optimized reaction conditions
After characterization of Fe3O4@SiO2-Ag catalyst, the oxidation of benzyl alcohol was studied as model for oxidant-free alcohols dehydrogenation with Fe3O4@SiO2-Ag catalyst. In fact, the oxidation of benzyl alcohol to benzaldehyde is a very popular model reaction for the study of catalytic oxidation of alcohols.Various reaction conditions were screened to optimize the catalytic process. As seen from Table 1, the reaction was conducted using silver-free Fe3O4@SiO2. The dehydrogenation reaction of benzyl alcohol gave only a trace amount of corresponding aldehyde (entry 1). Also, the AgNO3 precursor showed no catalytic activity under similar reaction conditions (entry 2). The use of Fe3O4@SiO2-Ag catalyst caused the reaction to performed (entry 3–8). This result shows that dehydrogenation of alcohol has been proceeded on surface of silver nanoparticle. Subsequently, the influence of the amount of Ag on oxidation was investigated. As seen in Table 1, it was found that the amount of catalyst could remarkably promote conversion of benzyl alcohol. As shown in Table 1, the conversion of alcohol was 80% as the amount of Ag was 0.05 mol% (3.75 mg of catalyst). With the increase of the amount of Ag to 0.20 mol% (15 mg of catalyst), the conversion gave to 98%. When the amount of catalyst was increased from 0.20 mol% (15 mg of catalyst) to 0.5 mol% (37.5 mg of catalyst), the conversion of alcohol and selectivity for aldehyde did not change remarkably. These results show that 0.20 mol% (15 mg of catalyst) catalyst is sufficient for oxidant-free dehydrogenation of alcohols to corresponding aldehydes.
Under the optimized conditions, the oxidation of various alcohols to their corresponding products was investigated. The reaction of benzyl alcohols containing different electron-withdrawing and electron-donating substituent groups on the aromatic ring was studied which gave the corresponding benzaldehydes in good to excellent yield (Table 2, entries 3–8). To investigate the steric effect on the oxidation reaction, 2-substituted benzyl alcohols were also investigated which gave well yields (Table 2, entry 3).
In most cases, alcohol was converted to corresponding carbonyl compound in excellent yield. Therefore, these results show the good ability of this protocol in oxidation of different types of alcohols. Unfortunately, aliphatic alcohols were not oxidized sufficiently under optimized condition. Therefore, the amount of catalyst was increased to 0.40 mol% (30 mg) and the reaction time increased to 48 h. The increase of reaction time and the amount of catalyst cause the increase of conversion. For example, in the case of 1-octanol, the increase of catalyst amount from 0.20 to 0.40 mol% led to increase of yield from 31% to 55%.
In the oxidation of cyclohexane-1,2-diol, one of the hydroxyl groups was only oxidized in specified time in Table 2. With the increase of time to 72 h, another hydroxyl group was also oxidized to carbonyl group.
The chemoselectivity of the presented protocol was investigated in the selective oxidation of cinnamyl alcohol containing double bond. The results showed that the double bond remained intact during the conversion of cinnamyl alcohol to cinnamaldehyde and only small amounts of 3-phenyl-1-propanol and 3-phenyl hydrocinnamaldehyde were produced. The present protocol also offers a good catalytic system for the oxidation of benzoin (entry 1), with no cleavage of carbon–carbon bonds which is generally observed in other conventional methods.
A reaction mechanism is presented in Scheme 2 in the case of benzyl alcohol dehydrogenation as an example. In the first step, benzyl alcohol interacts with the Si–OH groups in the Fe3O4@SiO2-Ag surface by hydrogen bond interaction. In the next step, C–H bond cleavage is performed on the Ag nanoparticles on the Fe3O4@SiO2 surface and then proton abstraction from Si–OH group on the surface is performed to form H2 adsorbed on Ag nanoparticles. At this time, the produced acetaldehyde is adsorbed on the silica surface by hydrogen bond interaction. In the last step, the H2 and benzaldehyde molecules are desorbed and the active sites are regenerated in the surface.
The recycling and recovery of the supported catalysts are a very important issue in term of application. To clarify this issue, the recycling property of the Fe3O4@SiO2-Ag catalyst was investigated in benzyl alcohol oxidation at 1 mmol of substrate. After completion of the first oxidation reaction of benzyl alcohol to corresponding benzaldehyde under optimized condition, the catalyst was magnetically isolated, washed with EtOH, and then placed into a fresh reaction mixture. Under the described conditions, the catalyst could be reused at least eight times without any change in activity (Fig. 5).
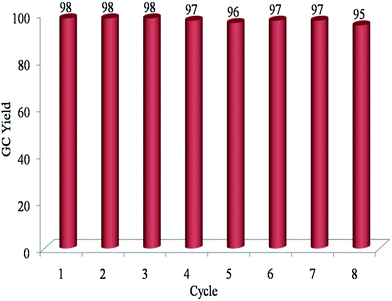 | ||
| Fig. 5 The recycling experiment of catalyst in the dehydrogenation of benzyl alcohol under optimized condition; the oxidation reaction was quenched after 24 h at each step. | ||
To investigate the catalyst leaching, we completely terminated the reaction by a removal of catalyst from the reaction mixture at 50% yield of benzaldehyde and the solution was kept back for oxidation under the same conditions. Our results indicated that the yield of product did not increase. To understand the amount of Ag leached from catalyst, inductively coupled plasma-atom emission spectrometer (ICP-AES) analysis of the liquid phase was investigated. The results of ICP-AES analysis showed no detectable amount of Ag in the liquid phase. This indicates that Ag nanoparticles are not leached from the catalyst surface during the oxidation process.
Conclusion
In summary, we have developed a new robust magnetically separable nanocatalyst for the oxidant-free dehydrogenation of various alcohols by using the silver deposited on magnetic nanoparticle (Fe3O4@SiO2-Ag). Under the optimized reaction conditions, a wide range of alcohols with different steric/electronic effects and containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds could be converted into their corresponding carbonyl compounds with high chemoselectivity. Because of the magnetic nature of the catalyst, it can be separated using an external magnet which eliminates the requirement of catalyst filtration after completion of the reaction. The catalyst was easily separated and reused for at least eight cycles with high activity.
C double bonds could be converted into their corresponding carbonyl compounds with high chemoselectivity. Because of the magnetic nature of the catalyst, it can be separated using an external magnet which eliminates the requirement of catalyst filtration after completion of the reaction. The catalyst was easily separated and reused for at least eight cycles with high activity.
Notes and references
- W. Li, A. Wang, X. Liu and T. Zhang, Appl. Catal., A, 2012, 433–434, 146 CrossRef CAS PubMed.
- V. Cortés Corberán, M. E. González-Pérez, S. Martínez-González and A. Gómez-Avilés, Appl. Catal., A, 2014, 474, 211 CrossRef PubMed.
- J. B. Arterburn, Tetrahedron, 2001, 57, 9765 CrossRef CAS.
- G. Tojo and M. Fernández, Oxidation of Alcohols to Aldehydes and Ketones, Springer, New York, 2010 Search PubMed.
- M. Hundlucky, Oxidations in Organic Chemistry, American Chemical Society, Washington, DC, 1990 Search PubMed.
- J. Wang, X. Lang, B. Zhaorigetu, M. Jia, J. Wang, X. Guo and J. Zhao, ChemCatChem, 2014, 6, 1737 CrossRef CAS.
- T. Nishimura, T. Onoue, K. Ohe and S. Uemura, J. Org. Chem., 1999, 64, 6750 CrossRef CAS PubMed.
- T. Wang, C. Xiao, L. Yan, L. Xu, J. Luo, H. Shou, Y. Kou and H. Liu, Chem. Commun., 2007, 4375 RSC.
- M. B. Gawande, O. M. N. D. Teodoro, A. Rathi, I. D. Nogueira and P. S. Branco, ChemPlusChem, 2012, 77, 865 CrossRef CAS.
- J. H. Choi, N. Kim, Y. J. Shin, J. H. Park and J. Park, Tetrahedron Lett., 2004, 45, 4607 CrossRef CAS PubMed.
- H. K. Kwong, P. K. Lo, K. C. Lau and T. C. Lau, Chem. Commun., 2011, 47, 4273 RSC.
- B. Karimi, A. Biglari, J. H. Clark and V. Budarin, Angew. Chem., Int. Ed., 2007, 46, 7210 CrossRef CAS PubMed.
- F. Su, S. C. Mathew, G. Lipner, X. Fu, M. Antonietti, S. Blechert and X. Wang, J. Am. Chem. Soc., 2010, 132, 16299 CrossRef CAS PubMed.
- F. Shi, M. K. Tse and M. Beller, Chem.–Asian J., 2007, 2, 411 CrossRef CAS PubMed.
- C. Parmeggiani and F. Cardona, Green Chem., 2012, 14, 547 RSC.
- T. C. Johnson, D. J. Morris and M. Wills, Chem. Soc. Rev., 2010, 39, 81 RSC.
- N. Armaroli and V. Balzani, ChemSusChem, 2011, 4, 21 CrossRef CAS PubMed.
- V. L. Sushkevich, I. I. Ivanova and E. Taarning, ChemCatChem, 2013, 5, 2367 CrossRef CAS.
- M. Nielsen, A. Kammer, D. Cozzula, H. Junge, S. Gladiali and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 9593 CrossRef CAS PubMed.
- R. Kawahara, K. I. Fujita and R. Yamaguchi, Angew. Chem., Int. Ed., 2012, 51, 12790 CrossRef CAS PubMed.
- K. Shimizu, K. Sugino, K. Sawabe and A. Satsuma, Chem.–Eur. J., 2009, 15, 2341 CrossRef CAS PubMed.
- I. Geukens, F. Vermoortele, M. Meledina, S. Turner, G. V. Tendeloo and D. E. De Vos, Appl. Catal., A, 2014, 469, 373 CrossRef CAS PubMed.
- K. Shimizu, K. Ohshima and A. Satsuma, Chem.–Eur. J., 2009, 15, 9977 CrossRef CAS PubMed.
- T. Mitsudome, Y. Mikami, H. Funai, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., 2008, 120, 144 (Angew. Chem., Int. Ed., 2008, 47, 138) CrossRef.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC.
- R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2013, 49, 752 RSC.
- A. Bayat, M. Shakourian-Fard and M. Mahmoodi Hashemi, Catal. Commun., 2014, 52, 16 CrossRef CAS PubMed.
- S. Santra, H. S. Yang, D. Dutta, J. T. Stanley, P. H. Holloway, W. H. Tan, B. M. Moudgil and R. A. Mericle, Chem. Commun., 2004, 24, 2810 RSC.
- X. Zhang, H. Niu, J. Yan and Y. Cai, Colloids Surf., A, 2011, 375, 186 CrossRef CAS PubMed.
- S. Akbayrak, M. Kaya, M. Volkan and S. Özkar, Appl. Catal., B, 2014, 147, 387 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |