DOI:
10.1039/C4RA15391J
(Paper)
RSC Adv., 2015,
5, 19734-19742
Effects of changing the M2+ cation on the crystal structure and optical properties of divalent samarium-doped MAl2Si2O8 (M = Ca, Sr, Ba)
Received
28th November 2014
, Accepted 5th February 2015
First published on 5th February 2015
Abstract
In order to investigate the effects of changing the cation on crystal structure and optical properties of divalent samarium-doped MAl2Si2O8, the Sm2+ doped triclinic CaAl2Si2O8 (CASO), monoclinic SrAl2Si2O8 (SASO) and BaAl2Si2O8 (M-BASO) as well as hexagonal BaAl2Si2O8 (H-BASO) compounds have been synthesized using a polymerizable-complex technique under a reducing atmosphere. At room temperature, under N-UV or blue excitation, Sm2+ doped SASO, M-BASO and H-BASO showed strong red emission, which widens the group of novel red-emitting materials. The emission bands of Sm2+ consist of a broad 4f–5d transition band and sharp f–f transition lines. The correlation between the crystal structure and the 4f–4f transition emission intensity of Sm2+ as well as the excitation peaks of the 5d energy level is discussed in detail, and the theoretical analysis is in good agreement with our experimental results. These are essential to understand the relationship between the crystal structure and luminescence properties, which are fundamental to design luminescent materials of Sm systems.
1. Introduction
The alkaline-earth feldspars MAl2Si2O8 (M = Ca, Sr, Ba) were reported to be very suitable host lattices for luminescent divalent rare earth ions, especially Eu2+ ions.1–8 Among the various rare earth ions, Sm2+ doped inorganic materials have found important applications in lighting, display and persistent spectral hole-burning (PSHB).9–13 Sm2+ ion doped inorganic materials have attracted more and more attention because they have the advantages of 4f–5d broad excitation and emission bands as well as the sharp f–f transition emission.9–14 Sm2+ generally has a broad continuum excitation band in the range from 200 to 500 nm and its emission is composed of the 4f–5d broad band and deep red 4f6–4f6 sharp emission. However, optical properties of Sm2+ doped alkaline-earth feldspars MAl2Si2O8 (M = Ca, Sr, Ba) have not been reported.
On the other hand, the luminescence of divalent samarium ions is sensitive to the crystalline environment of the lattice site that they occupy.15 The relative position between the lowest-lying 4f55d1 excited states and the 4f6 (5D0) level governs whether the emission displays the 4f6 → 4f6 sharp line and/or 4f55d1 → 4f6 broad band. It has been reported that the 4f–5d excited broad band is dependent on many factors such as the crystal field strength, the coordination number and the covalence of the bonds.10 Understanding the relationship between these factors and the 4f–5d transition of Sm2+ is of great importance due to the potential technological applications as functional photonic materials. We will choose the systematic alkaline-earth feldspars MAl2Si2O8 (M = Ca, Sr, Ba) as the host lattices to investigate the effects of the crystal structure on the 4f–5d excitation band due to their characteristic structural properties. In these host lattices, firstly, the number of the M2+ site occupied by Sm2+ is different. In CaAl2Si2O8, Ca2+ includes four different 2i sites,16 but only one kind of M2+ (Sr2+ or Ba2+) site exists in the SASO,17 M-BASO18 and H-BASO.4 So the effects of the number of the occupied sites on the 4f–5d transition can be obtained. Secondly, the symmetry of the M2+ is different. The Ba2+ site of H-BASO is central symmetry while the others are non-central symmetry. Thus the effects of the symmetry on the photoluminescence properties of Sm2+ can be shown. Thirdly, the SASO is monoclinic and isostructural with the M-BASO. They are similar to each other except the different cation site. So the effects of the cation ions on the 4f–5d of Sm2+ can be obtained. Fourthly, the H-BASO and the M-BASO are allomorphism. They are different in crystalline form without change in chemical constitution. So the effects the crystal structure with the same chemical constitution on the f–d transition peak can be investigated.
In order to obtain more exact photoluminescence data and high-efficiency phosphor emission, it is necessary to utilize solution-based techniques for synthesis. We used pechini-type sol–gel process19 (PSG, also known and called as polymerizable-complex technique) to synthesize the Sm doped MAl2Si2O8 (M = Ca, Sr, Ba). If precursor powders are prepared using PSG, all the component ions can be evenly dispersed in the precursors, leading to the formation of high-purity phosphors with a uniform dispersion of Sm ions activators.
In our paper, the Sm2+ doped triclinic CaAl2Si2O8 (CASO), monoclinic SrAl2Si2O8 (SASO) and BaAl2Si2O8 (M-BASO) as well as hexagonal BaAl2Si2O8 (H-BASO) have been synthesized using polymerizable-complex technique under reducing atmosphere. The photoluminescence properties of Sm2+ in the systematic host lattices have been reported. Furthermore, the important chemical bonds such as the environmental factor (he) and parameter (FC) were calculated using the dielectric theory of complex crystals. The effects of the crystal structure on the 4f–5d transition and f–f emission intensity of Sm2+ were discussed.
2. Experimental section
2.1. Sample preparation
Unless otherwise stated, all reagents were purchased from Sigma-Aldrich. The MAl2Si2O8 (M = Ca, Sr, Ba) powder samples were prepared by a pechini-type sol–gel process (PSG, also known and called as polymerizable-complex technique), which can give the highest possible homogeneity for the material.19 The doping level of Sm ions was 5.0 mol% in MAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm. The mixtures were heated in a hydrogen–nitrogen (5% H2 + 95% N2) atmosphere.
Sm. The mixtures were heated in a hydrogen–nitrogen (5% H2 + 95% N2) atmosphere.
The raw materials were Sm2O3, Ca(NO3)2·4H2O, Ba(NO3)2, Sr(NO3)2, Al(NO3)3·9H2O, tetraethyl orthosilicate (Si(OC2H5)4, TEOS) and citric acid as the chelating agent. The 0.05 M Sm(NO3)3 solution was prepared by dissolving Sm2O3 in the diluted HNO3 and adding appropriate volume of de-ionized water. The 0.25 M M(NO3)2 (M = Ca, Sr, Ba), Al(NO3)3 solutions and 1 M citric acid solution were prepared using the de-ionized water. Briefly, a stoichiometric volumes of M(NO3)2 (M = Ca, Sr, Ba) and Al(NO3)3 solutions were dissolved in an aqueous solution of citric acid (citric acid–M2+ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio) under vigorous stirring to form solution “A”. Then TEOS was dissolved using some ethanol under stirring to form solution “B”. Then A and B were mixed together and stirred for several hours at room temperature. The resultant mixture was heated up to 120 °C and kept at the temperature for 4 h to produce solid gels. The solid gels were prefired at 1200 °C for 4 h. After being fully ground, Sm
1, molar ratio) under vigorous stirring to form solution “A”. Then TEOS was dissolved using some ethanol under stirring to form solution “B”. Then A and B were mixed together and stirred for several hours at room temperature. The resultant mixture was heated up to 120 °C and kept at the temperature for 4 h to produce solid gels. The solid gels were prefired at 1200 °C for 4 h. After being fully ground, Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M-BASO (M = Ca, Sr) samples were annealed at 1400 °C in the reducing atmosphere for 6 h and single hexagonal and monoclinic phases of Sm
M-BASO (M = Ca, Sr) samples were annealed at 1400 °C in the reducing atmosphere for 6 h and single hexagonal and monoclinic phases of Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BASO were obtained between 1350 to 1650 °C in the reducing atmosphere for 6 h.
BASO were obtained between 1350 to 1650 °C in the reducing atmosphere for 6 h.
2.2. Characterizations
The phase purity of the as prepared phosphor was checked by powder X-ray diffraction (XRD) analysis by using a D/MAX 2500 instrument (Rigaku) with a Rint 2000 wide angle goniometer and Cu Kα1 radiation (λ = 1.54056 Å) at 40 kV and 100 mA. The measurements of photoluminescence (PL) and photoluminescence excitation (PLE) spectra were performed by using a fluorescence spectrophotometer (Photon Technology International) equipped with a 60 W Xe-arc lamp as the excitation light source. All the measurements were taken at room temperature.
3. Theoretical method
The dielectric theory of complex crystals was used to calculate the chemical bond parameters of inorganic compounds quantitatively.20–24 In this theory, the complex crystals with crystal formula Aa11Aa22⋯AaiiBb11Bb22⋯Bbjj can be written as a linear combination of the sub-formula of various binary crystals when the crystal structure is known. The sub-formula of any type of chemical bond A–B in the multi-bond crystal Aa11Aa22⋯AaiiBb11Bb22⋯Bbjj… can be expressed by the following formula:| |
 | (1) |
where| |
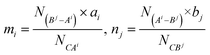 | (2) |
and the bond sub-formula equation is given by:| |
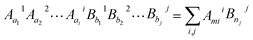 | (3) |
where Aaii, Bbjj stands for the different constituent elements or different sites of the same element in the crystal formula, and ai, bj represents the number of the corresponding element. N(Bj−Ai) is the number of Bj ions in the coordination group of a Ai ion, and NCAi represents the nearest coordination number of Ai ion. This means that the complex crystal is decomposed into the sum of different binary crystals like AmiiBnjj.
Once the different binary crystals are obtained, the covalency of any μ type binary bond can be defined as:25
| |
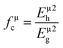 | (4) |
where
| | |
(Eμg)2 = (Eμh)2 + (Cμ)2
| (5) |
and
| |
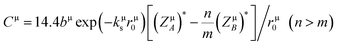 | (7) |
| |
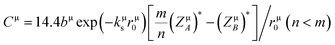 | (8) |
here,
dμ is the bond distance (in Å) and
rμ0 is half of the bond distance.
kμs is the Thomas–Fermis screening wavenumber of valence electrons and
αB is the Bohr radius.
bμ is proportional to the square of the average coordination number. (
ZμA)
* and (
ZμB)
* are the number of effective valence electrons of the cation and anion in the μ type bond, respectively.
For the chemical bond of type μ, the polarizable coefficient αμ0 can be obtained from the Lorentz–Lorenz equation
| | |
(εμ − 1)/(εμ − 2) = (4π/3)αμ0
| (9) |
Hence, the polarizability of the chemical bond volume (Å3) is given by:
More detail calculation process can be referred to the ref. 25.
4. Result and discussion
4.1. Crystal phase formation
Fig. 1 shows X-ray diffraction (XRD) patterns of Sm-doped MAl2Si2O8 samples prepared in reducing atmosphere. It is clear that all the diffraction peaks of four Sm-doped compounds can be readily indexed to the corresponding standard data for triclinic phase of CaAl2Si2O8 (JCPDS 48-1454), monoclinic phase of SrAl2Si2O8 (JCPDS 38-1454) and BaAl2Si2O8 (JCPDS 38-1450) as well as the hexagonal phase of BaAl2Si2O8 (JCPDS 28-0124), respectively. No other impurities can be detected. In addition, the refined crystallographic unit cell parameters of the calcined products were calculated using the software Jade 5.0 and listed in Table 1.
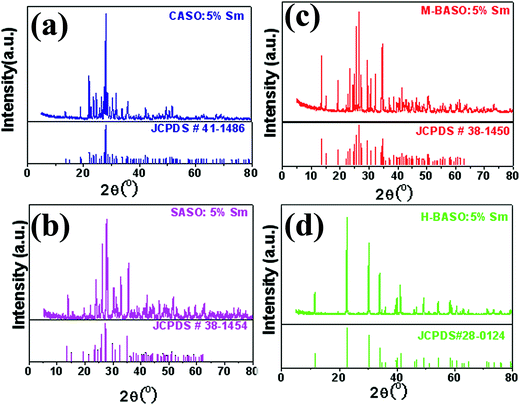 |
| | Fig. 1 XRD patterns of Sm-doped MAl2Si2O8 samples prepared in reducing atmosphere. (a) CaAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm; (b) SrAl2Si2O8 Sm; (b) SrAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm; (c) monoclinic BaAl2Si2O8 Sm; (c) monoclinic BaAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm; (d) hexagonal BaAl2Si2O8 Sm; (d) hexagonal BaAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm. Sm. | |
Table 1 Unit cell parameters of Sm-doped MAl2Si2O8 samples
| Compound |
a (Å) |
b (Å) |
c (Å) |
α (°) |
β (°) |
γ (°) |
| Ref. 4. Ref. 18. Ref. 17. Ref. 16. |
H-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm 5% Sm |
5.2942 |
5.2942 |
7.7836 |
90 |
90 |
120 |
| Pure H-BASOa |
5.2955 |
5.2955 |
7.7817 |
90 |
90 |
120 |
M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm 5% Sm |
8.6316 |
13.0444 |
14.4053 |
90 |
115.11 |
90 |
| Pure M-BASOb |
8.6268 |
13.045 |
14.408 |
90 |
115.22 |
90 |
SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm 5% Sm |
8.3705 |
12.9671 |
14.2559 |
90 |
115.25 |
90 |
| Pure SASOc |
8.392 |
12.967 |
14.260 |
90 |
115.43 |
90 |
CASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm 5% Sm |
8.16785 |
12.87079 |
14.17677 |
93.2473 |
115.731 |
91.1248 |
| Pure CASOd |
8.173 |
12.869 |
14.165 |
93.113 |
115.913 |
91.261 |
4.2. Photoluminescence properties
The photoluminescence properties of Sm2+ in different host lattices are quite different because of their different environmental factor. Divalent Sm2+ has the 4f6 electron configuration. Under irradiation with UV to blue light, it can be excited into the 4f55d1 continuum, from which the ions rapidly relax to the lowest excited state.9–13 Just as the Eu2+ or Ce3+ ions, the f–d luminescence wavelength of phosphors employing Sm2+ changes greatly with the type of the host crystal.1,26
4.2.1. Characteristic luminescence of Sm2+ in MAl2Si2O8 (M = Ca, Sr, Ba)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Sm. Fig. 2 shows the excitation and emission spectra of Sm doped CASO, SASO, M-BASO and H-BASO samples at room temperature with the initial samarium concentration of 5 mol%. Different excitation wavelength produces different photoluminescence spectra. For CASO
Sm. Fig. 2 shows the excitation and emission spectra of Sm doped CASO, SASO, M-BASO and H-BASO samples at room temperature with the initial samarium concentration of 5 mol%. Different excitation wavelength produces different photoluminescence spectra. For CASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, Fig. 2(a) shows the broad excitation band monitored by 689 nm with the peak at 375 nm. In the emission spectra under the excitation at 375 nm, a weaker 5D0 → 7F0 transition of Sm2+ at 698.5 nm can be found (Fig. 2(b)). Unusual luminescence of Sm3+ in CASO can be monitored. As can be shown in Fig. 2(b), the sharp emission lines at 565, 601, 648 nm come from the f–f transitions of Sm3+, which can be assigned to 4G5/2 → 6H5/2, 4G5/2 → 6H7/2, 4G5/2 → 6H9/2, respectively. It indicates the existence of Sm3+ in CASO.
Sm, Fig. 2(a) shows the broad excitation band monitored by 689 nm with the peak at 375 nm. In the emission spectra under the excitation at 375 nm, a weaker 5D0 → 7F0 transition of Sm2+ at 698.5 nm can be found (Fig. 2(b)). Unusual luminescence of Sm3+ in CASO can be monitored. As can be shown in Fig. 2(b), the sharp emission lines at 565, 601, 648 nm come from the f–f transitions of Sm3+, which can be assigned to 4G5/2 → 6H5/2, 4G5/2 → 6H7/2, 4G5/2 → 6H9/2, respectively. It indicates the existence of Sm3+ in CASO.
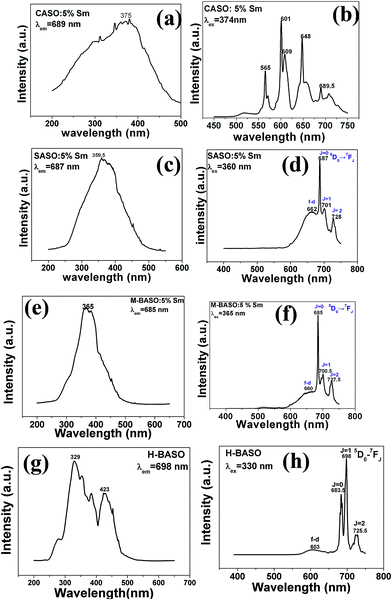 |
| | Fig. 2 Photoluminescence emission and excitation spectra of Sm-doped MAl2Si2O8 (M = Ba, Sr, Ca) samples related to the luminescence centre ions Sm2+ at room temperature. | |
Fig. 2(d), (f) and 2(h) show that Sm doped SASO, M-BASO and H-BASO samples exhibit efficient deep red emission under irradiation with UV light. The excitation and emission peak positions are listed in Table 2. For SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, the excitation spectrum (Fig. 2(c), λex = 687 nm) consists of a broad band from 250 to 500 nm with a maximum at 359.5 nm. This is assigned to 4f–5d transition of Sm2+. As is shown in Fig. 2(d), under the excitation into the 4f55d1 states with the wavelength at 360 nm, the obtained emission spectra of SASO
Sm, the excitation spectrum (Fig. 2(c), λex = 687 nm) consists of a broad band from 250 to 500 nm with a maximum at 359.5 nm. This is assigned to 4f–5d transition of Sm2+. As is shown in Fig. 2(d), under the excitation into the 4f55d1 states with the wavelength at 360 nm, the obtained emission spectra of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm show a broad band emission from 600 to 750 nm overlapped with the f–f transitions of Sm2+. The peak of the broad band emission is 662 nm assigned to f–d transition of Sm2+. The sharp emission bands consists of three groups of lines at 687, 702 and 727 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The dominant line is about 687 nm (5D0 → 7F0 transition of Sm2+ ions), which shows that Sm2+ ions occupy the crystallographic sites without central symmetry in the host.
Sm show a broad band emission from 600 to 750 nm overlapped with the f–f transitions of Sm2+. The peak of the broad band emission is 662 nm assigned to f–d transition of Sm2+. The sharp emission bands consists of three groups of lines at 687, 702 and 727 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The dominant line is about 687 nm (5D0 → 7F0 transition of Sm2+ ions), which shows that Sm2+ ions occupy the crystallographic sites without central symmetry in the host.
Table 2 The excitation and emission peak positions of Sm2+ in the samples MAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm (M = Ba, Sr, Ca) (“*” indicates the strongest peak position of the excitation and emission spectra.)
5% Sm (M = Ba, Sr, Ca) (“*” indicates the strongest peak position of the excitation and emission spectra.)
| Sample |
Excitation (f → d) (nm) |
d → f |
Emission (nm) f–f transition (5D0 → 7FJ) |
| J = 0 |
J = 1 |
J = 2 |
CASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
375* |
— |
689.5* |
— |
— |
SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
359.5* |
662 |
687* |
702 |
727 |
M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
365* |
660 |
685* |
700.5 |
727.5 |
H-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
329* |
603 |
683.5 |
698* |
725.5 |
| 423* |
The excitation and emission spectra of M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm show in Fig. 2(e) and (f). The broad excitation band with the peak at 365 nm extends from 250 to 500 nm. And the broad f–d transition emission of Sm2+ ranges from 600 to 750 nm with the peak at 660 nm. The sharp emission bands consists of three groups of lines at 685, 700.5 and 727.5 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The optical properties of M-BASO
Sm show in Fig. 2(e) and (f). The broad excitation band with the peak at 365 nm extends from 250 to 500 nm. And the broad f–d transition emission of Sm2+ ranges from 600 to 750 nm with the peak at 660 nm. The sharp emission bands consists of three groups of lines at 685, 700.5 and 727.5 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The optical properties of M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm are similar to those of SASO
Sm are similar to those of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm. This is because that the crystal structure of M-BASO is close to that of SASO
Sm. This is because that the crystal structure of M-BASO is close to that of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, thus crystal environment of Sm2+ in M-BASO is near to SASO
Sm, thus crystal environment of Sm2+ in M-BASO is near to SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm. However, compared with that of SASO
Sm. However, compared with that of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, a red-shift of the excitation for M-BASO
Sm, a red-shift of the excitation for M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm can be found and the reason will be discussed in the discussion part.
Sm can be found and the reason will be discussed in the discussion part.
As can be shown in Fig. 2(g) and (h), the sample H-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm shows different excitation and emission spectra. The spectra are quite different with the photoluminescence properties of Sm doped SASO and M-BASO samples. Two dominate excitation peaks at 329 nm and 423 nm can be found, which can be assigned to the 4f–5d transition of Sm2+. The emission bands consist of a broad band emission from 550 to 650 nm with the peak at 603 nm. The sharp emission bands consists of three groups of lines at 683.5, 698 and 725.5 nm correspond to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The strongest emission is at 698 (5D0 → 7F1 of Sm2+), which indicates that the Sm2+ ions occupy the crystallographic sites with central symmetry in the host.
Sm shows different excitation and emission spectra. The spectra are quite different with the photoluminescence properties of Sm doped SASO and M-BASO samples. Two dominate excitation peaks at 329 nm and 423 nm can be found, which can be assigned to the 4f–5d transition of Sm2+. The emission bands consist of a broad band emission from 550 to 650 nm with the peak at 603 nm. The sharp emission bands consists of three groups of lines at 683.5, 698 and 725.5 nm correspond to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The strongest emission is at 698 (5D0 → 7F1 of Sm2+), which indicates that the Sm2+ ions occupy the crystallographic sites with central symmetry in the host.
Fig. 3 shows the CIE (commission International de I'Ecairage 1931) chromaticity coordinates of Sm doped CASO, SASO, M-BASO and H-BASO. For Sm-doped CASO, the emission light excited at 375 nm shows orange-red because it mainly comes from the emission of Sm3+. For Sm doped SASO, M-BASO and H-BASO, as can be shown in Fig. 2 and Table 3, excited at N-UV light, their CIE values are B (0.680, 0.319), C (0.635, 0.350) and D (0.616, 0.357), respectively. All of them yield strong deep red emission, which may be used as the red phosphors in the phosphors-converted light-emitting diodes.
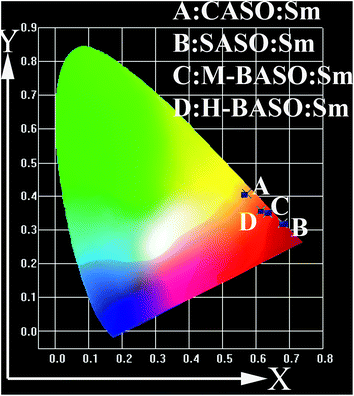 |
| | Fig. 3 CIE (commission International de I'Ecairage 1931) chromaticity coordinates of Sm doped CASO, SASO, M-BASO and H-BASO. | |
Table 3 CIE values of Sm doped CASO, SASO, M-BASO and H-BASO
| Compound |
CIE (excitation wavelength) |
CASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
(0.573, 0.407) |
| (374 nm) |
SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
(0.680, 0.319) |
| (360 nm) |
M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
(0.635, 0.350) |
| (365 nm) |
H-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm Sm |
(0.616, 0.357) |
| (330 nm) |
4.2.2. Crystal structure and spectra of Sm doped MAl2Si2O8 (M = Ca, Sr, Ba).
4.2.2.1. The relationship between the crystal structure and the f–f transition intensity of Sm2+. From the above analysis, it has been clearly shown that the f–d transition energy of Sm doped SASO, M-BASO and H-BASO can produce f–d transition emission accompanied by some strong f–f transitions of Sm2+, however, the f–f emission intensity are quite different. Their comparison of the emission intensity under the excitation of 360 or 460 nm can be shown in Fig. 4. The clear order of the strongest f–f emission intensity of Sm2+ is H-BASO ≫ M-BASO > SASO > CASO. We will make our focus on the spectra analysis of Sm doped H-BASO, M-BASO and SASO to try to find out the relationship between the crystal structure and their PL properties because their strong f–d and f–f transition of Sm2+ at room temperature.
 |
| | Fig. 4 Comparison emission intensity of Sm doped CASO, SASO, M-BASO and H-BASO under the f–d transition energy excitation. | |
As is well known, the relative intensity of f–f transitions is influenced by the crystalline environment due to the different symmetry of the occupied sites.9,10,27–30 The crystal structure of MASO (M = Ca and Ba) has been shown in Fig. 5. In the Sm doped MASO (M = Ca and Ba) systematic samples, the Sm ions occupy the site of M2+ ions, therefore, the f–f transition intensity is mainly influenced by the symmetry of the M2+ sites. Just as the Eu3+ emission, the intensities of different 5D0–7FJ transitions of Sm2+ depend on the local symmetry of the crystal field of the Sm2+ ions.9–13 The 5D0–7F0 transition is hypersensitive, while the 5D0–7F1 transition is insensitive to the crystal field environment. For instance, in a site with inversion symmetry, the 5D0–7F1 transition is dominant, while in a site without inversion symmetry, the 5D0–7F0 electronic transition becomes the strongest one. The intensity of 5D0–7F0 transition is much higher than that of 5D0–7F1, which is strong evidence that Sm2+ ions mainly occupy the lattice site without inversion symmetry.9 From the crystal structure and the skeleton construction of MASO (Fig. 5), we can see that only in the Sm-doped H-BASO sample, Sm occupy the centered site with six identity oxygens (O2), which is inversion symmetry, so the intensity of 5D0–7F1 transition of Sm2+ in H-BASO is the strongest among the four samples.
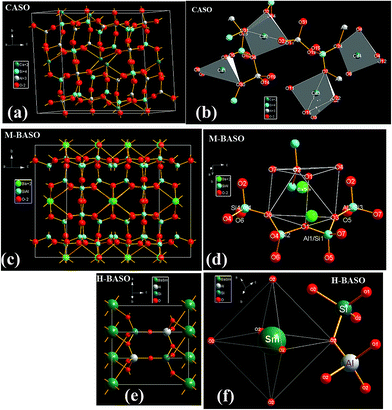 |
| | Fig. 5 Crystal structure of Sm doped-CASO, M-BASO and H-BASO. (a), (c) and (e): shape of the unit cell; (b), (d) and (f): Sm/Ca, Sm/Ba-centred skeleton construction with nearest neighbor atoms, respectively. The detail structure data of CASO, M-BASO, SASO and H-BASO can be referred to ref. 4 and 16–18, respectively. | |
The space groups of M-BASO and SASO are I12/c1 (no. 15).17,18 Both of the occupied sites of Ba2+ in M-BASO and Sr2+ in SASO are 8f. The symmetry of Ba2+ in M-BASO crystal is similar to that of Sr2+ in SASO crystal, but the intensity of 5D0–7F0 transition of Sm2+ of M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm is about 2 time stronger than that of SASO
Sm is about 2 time stronger than that of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, which can be shown in Fig. 4(b) and d. In their crystal structure, as can be shown in Fig. 5(c) and (d), there is one 8f site of Ba with seven different nearest neighbors atoms including two types of Ba–O1, one Ba–O2, one Ba–O3, one Ba–O4, one Ba–O7 and one Ba–O8 bonds. This type of symmetry produces the strong emission of the 5D0–7F0 transition of Sm2+. It has been found that the photoluminescence intensity ratio between 5D0 → 7F2 and 5D0 → 7F1 emission of Eu3+ (I(5D0 → 7F2)/I(5D0 → 7F1)) increases with increasing of the distortion degree of LnO6 or LnO7 polyhedron from that of an ideal octahedron. So, just as the Eu3+, the transition intensity ratio between 5D0–7F0 and 5D0–7F1 emission of Sm2+ (I(5D0 → 7F0)/I(5D0 → 7F1)) is influenced by the distortion degree of LnOn polyhedron from that of an ideal polyhedron.27,28 Their ratio values of I(5D0 → 7F0)/I(5D0 → 7F1) for SASO
Sm, which can be shown in Fig. 4(b) and d. In their crystal structure, as can be shown in Fig. 5(c) and (d), there is one 8f site of Ba with seven different nearest neighbors atoms including two types of Ba–O1, one Ba–O2, one Ba–O3, one Ba–O4, one Ba–O7 and one Ba–O8 bonds. This type of symmetry produces the strong emission of the 5D0–7F0 transition of Sm2+. It has been found that the photoluminescence intensity ratio between 5D0 → 7F2 and 5D0 → 7F1 emission of Eu3+ (I(5D0 → 7F2)/I(5D0 → 7F1)) increases with increasing of the distortion degree of LnO6 or LnO7 polyhedron from that of an ideal octahedron. So, just as the Eu3+, the transition intensity ratio between 5D0–7F0 and 5D0–7F1 emission of Sm2+ (I(5D0 → 7F0)/I(5D0 → 7F1)) is influenced by the distortion degree of LnOn polyhedron from that of an ideal polyhedron.27,28 Their ratio values of I(5D0 → 7F0)/I(5D0 → 7F1) for SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, M-BASO and H-BASO are about 2.21, 3.34 and 0.95, respectively. The distortion degree can be calculated using the standard deviation of environmental factor of the individual bond (EFSD) σ(hei), which can be expressed as below:
Sm, M-BASO and H-BASO are about 2.21, 3.34 and 0.95, respectively. The distortion degree can be calculated using the standard deviation of environmental factor of the individual bond (EFSD) σ(hei), which can be expressed as below:
| |
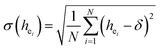 | (11) |
where
| | |
hei = (fμcαμb)1/2QμB
| (12) |
and
| |
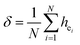 | (13) |
The related chemical parameters, such as the covalency fc, the polarizability of the chemical bond volume αb, and the present charge of the ligand in the binary crystals, are listed in Table 4. According to the eqn (11)–(13), their standard deviation of the seven M–O environmental factors (σ(hei)) of MO7 or MO6 polyhedron in the SASO, M-BASO and H-BASO can be calculated to be 0.03379, 0.07348 and 0 respectively. The σ(hei) value of M-BASO is 2.2 times larger than that of SASO. Generally, the I(5D0 → 7F0)/I(5D0 → 7F1) value of Sm2+ increases with increasing of σ(hei).27,28 Therefore, the I(5D0 → 7F0)/I(5D0 → 7F1) of Sm2+ in the crystal M-BASO is stronger than that in the crystal SASO or H-BASO, and I(5D0 → 7F0)/I(5D0 → 7F1) of H-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm is the lowest.
Sm is the lowest.
Table 4 The environmental factor of any individual bond between the centre-M2+ atom and its nearest coordination atom (hei) in the monoclinic crystals M-BASO and SASO, their standard deviation of the seven M–O environmental factors (σ(hei)) as well as the emission intensity ratio between 5D0 → 7F0 and 5D0 → 7F1  in the hexagonal crystal H-BASO and monoclinic crystals M-BASO and SASO
in the hexagonal crystal H-BASO and monoclinic crystals M-BASO and SASO
| Samples |
Central ion |
Bond type |
fc |
αb |
QB |
C.N. |
hei |
σ(hei) |

|
| SASO |
Sr2+ |
Sr–O1 |
0.0318 |
0.1810 |
1.1429 |
2 |
0.08671 |
0.03379 |
2.21 |
| Sr–O2 |
0.0696 |
0.3123 |
0.8571 |
1 |
0.12636 |
| Sr–O3 |
0.0650 |
0.5346 |
0.8571 |
1 |
0.15977 |
| Sr–O4 |
0.0639 |
0.6209 |
0.8571 |
1 |
0.17072 |
| Sr–O7 |
0.0646 |
0.5603 |
0.8571 |
1 |
0.16306 |
| Sr–O8 |
0.0650 |
0.5306 |
0.8571 |
1 |
0.15917 |
| M-BASO |
Ba2+ |
Ba–O1 |
0.0304 |
0.2402 |
1.1429 |
2 |
0.09766 |
0.07348 |
3.34 |
| Ba–O2 |
0.0655 |
0.4451 |
0.8571 |
1 |
0.14635 |
| Ba–O3 |
0.0626 |
0.6692 |
0.8571 |
1 |
0.20222 |
| Ba–O4 |
0.0625 |
0.6809 |
0.8571 |
1 |
0.17681 |
| Ba–O7 |
0.0628 |
0.6497 |
0.8571 |
1 |
0.17313 |
| Ba–O8 |
0.0629 |
0.6437 |
0.8571 |
1 |
0.17246 |
| H-BASO |
Ba2+ |
Ba–O2 |
0.1047 |
1.1695 |
1.000 |
6 |
— |
0 |
0.951 |
4.2.2.2. The relationship between the crystal structure and the f–d transition energy of Sm2+. Fig. 6 shows the comparisons of 4f–5d transition excitation spectra in Sm2+ in H-BASO, M-BASO and SASO. (The intensity of H-BASO is reduced 6 times; and that of SASO is enlarged 1.5 times.) The lowest band comes from the Sm doped H-BASO (red line). The broad bands of Sm doped M-BASO and SASO have a blue-shift comparing with that of H-BASO. The 4f–5d transition band of Sm-SASO (blue line) has a blue-shift comparing with that of M-BASO (black line).
 |
| | Fig. 6 The 4f–5d transition excitation spectra of Sm2+ in H-BASO, M-BASO and SASO. (The intensity of H-BASO is reduced 6 times; and that of SASO is enlarged 1.5 times.) The lowest band comes from the Sm doped H-BASO (red line). The broad bands of Sm doped M-BASO and SASO have a blue-shift comparing with that of H-BASO. The 4f–5d transition band of Sm-SASO (blue line) has a blue shift comparing with that of M-BASO (black line). | |
As is well known, the 4f–5d transition energy is greatly influenced by the crystalline environmental.21,22,26,31 This is because that the excited states such as 5d are not shielded from the surrounding electronic shell, so the 5d electron has a strong interaction with the neighboring anions in the compound. The interactions for the 4f55d configuration, which may be important for optical spectrum simulations, are as follows:32
| | |
H = H0 + HCoul(ff) + Hso(f) + Hcf(f) + Hcf(d) + Hso(d) + HCoul(fd)
| (14) |
where
H0 is the central-field interaction which includes the kinetic energy of electron and the Coulomb interaction between the 4f
5 core and the electrons;
HCoul(ff),
Hso(f) and
Hcf(f) are Coulomb, spin–orbit and crystal–field interactions for the 4f
5 core;
Hcf(d) and
Hso(d) are crystal field and spin orbit interactions for the single 5d electron;
HCoul(fd) is the Coulomb interaction between the 4f
5 core and the 5d electron. The order of magnitude of the energy splitting caused by these interactions is as follow:
| | |
H0〉[HCoul(ff),Hcf(d)]〉HCoul(fd)〉Hso(f)〉Hso(d)〉Hcf(f)
| (15) |
The interactions Hso(d) and Hcf(f) can be generally ignored. In our systematic samples the central ion is Sm2+ with the electron 4f6 configuration, the lowest 4f6 → 4f55d energy (Eexp(4f6 − 4f55d)) is determined by five parts: the energy centroid of the 5d orbital (EC), the Coulomb, spin–orbital interactions between 4f and 4f electrons (ECoul(ff),Eso(f)), the Coulomb interactions between 4f and 5d electrons (ECoul(fd)) and the effect of the crystal field (Ecf(d)). Fig. 7 shows a schematic energy diagram of the of 4f6 → 4f55d configuration of Sm2+ in any host lattice.
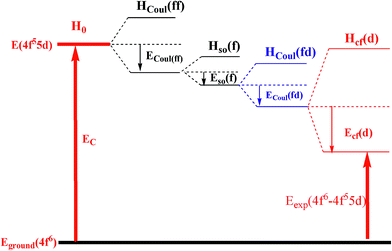 |
| | Fig. 7 A schematic energy diagram of 4f6 → 4f55d configuration of Sm2+ in any host lattice. | |
From the diagram (Fig. 7), the lowest 4f6 → 4f55d transition energy of Sm2+ can be obtained as follows:
| | |
Eexp(4f6 − 4f55d) = EC − ECoul(ff) − Eso(f) − ECoul(fd) − Ecf(d)
| (16) |
In our case, all of the luminescence centre ions are Sm2+, it is expected that ECoul(ff) and Eso(f) are unchanged in the MASO (M = Sr and Ba) crystals. The energy difference among Sm-doped SASO, H-BASO and M-BASO mainly comes from the difference of the other three parts of eqn (16): EC, ECoul(fd) and Ecf(d). EC and ECoul(fd) are related to the environmental factor he (ref. 31) and Ecf(d) is concerned with the environmental parameter FC.22 The values of he can be calculated using the below equations:
| |
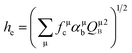 | (17) |
where
QμB stands for the presented charge of the nearest anion in the chemical bond, and
αμb is the polarizability of the chemical bond volume in the μ type of chemical bonds.
31 Also, the relationship between the environmental factor (
he) and the environmental factor of any individual bond between the centre atom and their nearest coordination atom (
hei) can be expressed by
| |
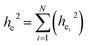 | (18) |
The values of the environmental parameter FC can be obtained as follows:
| |
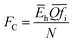 | (19) |
where
Ēh is the average homopolar part of the energy gap.
![[Q with combining macron]](https://www.rsc.org/images/entities/i_char_0051_0304.gif)
is the average present charge of the anions, and
![[f with combining macron]](https://www.rsc.org/images/entities/i_char_0066_0304.gif) i
i is the average bond ionicity between the central ion and the nearest neighbors.
According to the eqn (18) and (19), the values of the environmental factor he and the environmental parameter FC as well as their related chemical bond parameters are calculated and listed in Table 5. The he values of Sm doped H-BASO, M-BASO and SASO are 0.8573, 0.4028 and 0.3710, respectively. Generally, an increase of he leads to an decrease of the energy centroid of the 5d orbital (EC).31 Thus the order of their EC values is EC(H-BASO)〈EC(M-BASO)〈EC(SASO), and the order of the ECoul(fd) value is just reverse.26 It has been shown that the Ecf(d) increases with increasing of FC, so the order of the Ecf(d) value is Ecf(H-BASO)〈Ecf(SASO)〈Ecf(M-BASO). Finally, according to the eqn (16), it can be judged that among the three samples the lowest 4f–5d transition energy of Sm doped H-BASO is the lowest, which coincides with the experimental data (423 nm). For the other two crystal M-BASO and SASO, their difference between the environmental factors (he) (Δ(he) = 0.0318) is near to the difference between FC(Δ(he) = 0.0553). On the other hand, the magnitude of H0 is larger than others in eqn (14). Thus the difference of the Eexp(4f6 − 4f55d) value is mainly demined by the difference of EC, so the lowest 4f–5d energy of Sm-doped SASO is a little larger than that of Sm-doped M-BASO, which is consistent with the experimental result.
Table 5 The values of the corresponding crystal factors (FC) and environmental factor (he) of the central ions M2+ for H-BASO, SASO and M-BASO samples
| Samples |
Central ion |
Bond type |
Eh |
fi |
QB |
C.N. |
FC |
he |
| SASO |
Sr2+ |
Sr–O1 |
3.4944 |
0.9682 |
1.1429 |
2 |
0.4278 |
0.3710 |
| Sr–O2 |
4.3145 |
0.9304 |
0.8571 |
1 |
| Sr–O3 |
3.1692 |
0.9350 |
0.8571 |
1 |
| Sr–O4 |
2.9126 |
0.9361 |
0.8571 |
1 |
| Sr–O7 |
3.0862 |
0.9354 |
0.8571 |
1 |
| Sr–O8 |
3.1828 |
0.9350 |
0.8571 |
1 |
| M-BASO |
Ba2+ |
Ba–O1 |
2.9564 |
0.9696 |
1.1429 |
2 |
0.3725 |
0.4028 |
| Ba–O2 |
3.4860 |
0.9345 |
0.8571 |
1 |
| Ba–O3 |
2.7685 |
0.9374 |
0.8571 |
1 |
| Ba–O4 |
2.7417 |
0.9375 |
0.8571 |
1 |
| Ba–O7 |
2.8147 |
0.9372 |
0.8571 |
1 |
| Ba–O8 |
2.8292 |
0.9371 |
0.8571 |
1 |
| Ba–O2 |
2.9377 |
0.8953 |
1.000 |
6 |
| H-BASO |
Ba2+ |
Ba–O2 |
2.9377 |
0.8953 |
1.000 |
6 |
0.4384 |
0.8573 |
5. Conclusion
The Sm2+ doped triclinic CaAl2Si2O8 (CASO), monoclinic SrAl2Si2O8 (SASO) and BaAl2Si2O8 (M-BASO) as well as hexagonal BaAl2Si2O8 (H-BASO) have been synthesized using polymerizable-complex technique under reducing atmosphere. At room temperature, under the N-UV or blue excitation, Sm2+ doped SASO, M-BASO and H-BASO showed strong red emission. Their strongest emission spectra mainly come from the 5D0 → 7F0 transition of Sm2+ in the H-BASO crystal and the 5D0 → 7F1 transition of Sm2+ in SASO and M-BASO, respectively. The calculated standard deviation of environmental factor of the individual bond of Sm disclosed the reason why the emission intensity ratio (I(5D0 → 7F0)/I(5D0 → 7F1)) of Sm2+ in SASO is stronger than that in M-BASO and that of H-BASO is the lowest. The lowest excitation energy of 5d energy levels of Sm2+ have a blue-shift in the order H-BASO < M-BASO < SASO. The physical reason for spectral and energy level changes is analyzed in detail to be a comprehensive result from the shift of the energy centroid of the 5d orbital, the Coulomb interaction between 4f and 5d electrons, and the crystal-field splitting of 5d energy level. The theoretical analysis is in good agreement with our experimental result.
Acknowledgements
This work is financially supported by the National Natural Science Foundations of China (Grant no. 21301053).
References
- F. Clabau, A. Garcia, P. Bonville, D. Gonbeau, T. Le Mercier, P. Deniard and S. Jobic, J. Solid State Chem., 2008, 181, 1456 CrossRef CAS PubMed.
- W. B. Dai, J. Mater. Chem. C, 2014, 2, 3951 RSC.
- H. Guo, X. Y. Liu, F. Li, R. F. Wei, Y. L. Wei and C. Ma, J. Electrochem. Soc., 2012, 159, J223 CrossRef CAS PubMed.
- W. B. Im, Y.-I. Kim and D. Y. Jeon, Chem. Mater., 2006, 18, 1190 CrossRef CAS.
- M. Ma, D. Zhu, C. Zhao, T. Han, S. Cao and M. Tu, Opt. Commun., 2012, 285, 665 CrossRef CAS PubMed.
- Y. Wang, Z. Wang, P. Zhang, Z. Hong, X. Fan and G. Qian, Mater. Lett., 2004, 58, 3308 CrossRef CAS PubMed.
- C. Zhang, J. Yang, C. Lin, C. Li and J. Lin, J. Solid State Chem., 2009, 182, 1673 CrossRef CAS PubMed.
- Q. Zhang, X. Liu, Y. Qiao, B. Qian, G. Dong, J. Ruan, Q. Zhou, J. Qiu and D. Chen, Opt. Mater., 2010, 32, 427 CrossRef CAS PubMed.
- Y. Huang, W. Kai, K. Jang, H. S. Lee, X. Wang, Y. Zhang, D. Qin and C. Jiang, Mater. Lett., 2008, 62, 1913 CrossRef CAS PubMed.
- P. Mikhail, J. Hulliger, M. Schnieper and H. Bill, J. Mater. Chem., 2000, 10, 987 RSC.
- M. Nogami and Y. Abe, J. Appl. Phys., 1997, 81, 6351 CrossRef CAS PubMed.
- Z. Pei, Q. Su and J. Zhang, J. Alloys Compd., 1993, 198, 51 CrossRef CAS.
- X. Wang, H. Riesen, M. A. Stevens-Kalceff and R. P. Rajan, J. Phys. Chem. A, 2014, 118, 9445 CrossRef CAS PubMed.
- Q. Zeng, Z. Pei, S. Wang, Q. Su and S. Lu, Chem. Mater., 1999, 11, 605 CrossRef CAS.
- Q. Su, H. Liang, T. Hu, Y. Tao and T. Liu, J. Alloys Compd., 2002, 344, 132 CrossRef CAS.
- C. J. E. Kempster, H. D. Megaw and E. W. Radoslovich, Acta Crystallogr., 1962, 15, 1005 CrossRef CAS.
- P. Benna and E. Bruno, Am. Mineral., 2001, 86, 690 CAS.
- R. E. Newnham and H. D. Megaw, Acta. Crystallogr. A, 1960, 13, 303 CrossRef CAS.
- J. Lin, M. Yu, C. Lin and X. Liu, J. Phys. Chem. C, 2007, 111, 5835 CAS.
- F. M. Gao, J. L. He, E. D. Wu, S. m. Liu, D. l. Yu, D. c. Li, S. Y. Zhang and Y. J. Tian, Phys. Rev. Lett., 2003, 91, 015502 CrossRef.
- J. Shi and S. Zhang, J. Phys.: Condens. Matter, 2003, 15, 4101 CrossRef CAS.
- J. S. Shi, Z. J. Wu, S. H. Zhou and S. Y. Zhang, Chem. Phys. Lett., 2003, 380, 245 CrossRef CAS PubMed.
- Z. J. Wu and S. Y. Zhang, J. Phys. Chem. A, 1999, 103, 4270 CrossRef CAS.
- S. Y. Zhang, Chemical Bond Theory of Complex Structure Crystals on Dielectric Description
and Application, Science Publisher, Beijing, 2005 Search PubMed.
- L. Li and S. Y. Zhang, J. Phys. Chem. B, 2006, 110, 21438 CrossRef CAS PubMed.
- Z. Fu, S. Zhou and S. Zhang, J. Phys. Chem. B, 2005, 109, 14396 CrossRef CAS PubMed.
- L. Li, X. Liu, H. M. Noh, S. H. Park, J. H. Jeong and K. H. Kim, J. Alloys Compd., 2015, 620, 324 CrossRef CAS PubMed.
- L. Li, H. K. Yang, B. K. Moon, Z. Fu, C. Guo, J. H. Jeong, S. S. Yi, K. Jang and H. S. Le, J. Phys. Chem. C, 2009, 113, 610 CAS.
- J. G. Muller, J. Karthikeyan, P. Murugan and N. Lakshminarasimhan, J. Phys. Chem. C, 2014, 118, 19308 CAS.
- X. Zhang and H. J. Seo, J. Alloys Compd., 2011, 509, 2007 CrossRef CAS PubMed.
- J. S. Shi and S. Y. Zhang, J. Phys. Chem. B, 2004, 108, 18845 CrossRef CAS.
- S. Y. Zhang, Spectroscopy of Rare Earth Ions-Spectral property and Spectral Theory, Science Publisher, Beijing, 2008 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm. The mixtures were heated in a hydrogen–nitrogen (5% H2 + 95% N2) atmosphere.
Sm. The mixtures were heated in a hydrogen–nitrogen (5% H2 + 95% N2) atmosphere.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio) under vigorous stirring to form solution “A”. Then TEOS was dissolved using some ethanol under stirring to form solution “B”. Then A and B were mixed together and stirred for several hours at room temperature. The resultant mixture was heated up to 120 °C and kept at the temperature for 4 h to produce solid gels. The solid gels were prefired at 1200 °C for 4 h. After being fully ground, Sm
1, molar ratio) under vigorous stirring to form solution “A”. Then TEOS was dissolved using some ethanol under stirring to form solution “B”. Then A and B were mixed together and stirred for several hours at room temperature. The resultant mixture was heated up to 120 °C and kept at the temperature for 4 h to produce solid gels. The solid gels were prefired at 1200 °C for 4 h. After being fully ground, Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M-BASO (M = Ca, Sr) samples were annealed at 1400 °C in the reducing atmosphere for 6 h and single hexagonal and monoclinic phases of Sm
M-BASO (M = Ca, Sr) samples were annealed at 1400 °C in the reducing atmosphere for 6 h and single hexagonal and monoclinic phases of Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BASO were obtained between 1350 to 1650 °C in the reducing atmosphere for 6 h.
BASO were obtained between 1350 to 1650 °C in the reducing atmosphere for 6 h.
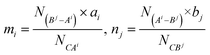
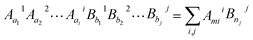
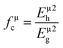
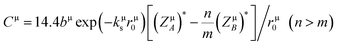
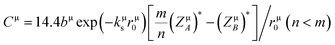

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm; (b) SrAl2Si2O8
Sm; (b) SrAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm; (c) monoclinic BaAl2Si2O8
Sm; (c) monoclinic BaAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm; (d) hexagonal BaAl2Si2O8
Sm; (d) hexagonal BaAl2Si2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm.
Sm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm
5% Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm
5% Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm
5% Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm
5% Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Sm. Fig. 2 shows the excitation and emission spectra of Sm doped CASO, SASO, M-BASO and H-BASO samples at room temperature with the initial samarium concentration of 5 mol%. Different excitation wavelength produces different photoluminescence spectra. For CASO
Sm. Fig. 2 shows the excitation and emission spectra of Sm doped CASO, SASO, M-BASO and H-BASO samples at room temperature with the initial samarium concentration of 5 mol%. Different excitation wavelength produces different photoluminescence spectra. For CASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, Fig. 2(a) shows the broad excitation band monitored by 689 nm with the peak at 375 nm. In the emission spectra under the excitation at 375 nm, a weaker 5D0 → 7F0 transition of Sm2+ at 698.5 nm can be found (Fig. 2(b)). Unusual luminescence of Sm3+ in CASO can be monitored. As can be shown in Fig. 2(b), the sharp emission lines at 565, 601, 648 nm come from the f–f transitions of Sm3+, which can be assigned to 4G5/2 → 6H5/2, 4G5/2 → 6H7/2, 4G5/2 → 6H9/2, respectively. It indicates the existence of Sm3+ in CASO.
Sm, Fig. 2(a) shows the broad excitation band monitored by 689 nm with the peak at 375 nm. In the emission spectra under the excitation at 375 nm, a weaker 5D0 → 7F0 transition of Sm2+ at 698.5 nm can be found (Fig. 2(b)). Unusual luminescence of Sm3+ in CASO can be monitored. As can be shown in Fig. 2(b), the sharp emission lines at 565, 601, 648 nm come from the f–f transitions of Sm3+, which can be assigned to 4G5/2 → 6H5/2, 4G5/2 → 6H7/2, 4G5/2 → 6H9/2, respectively. It indicates the existence of Sm3+ in CASO.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, the excitation spectrum (Fig. 2(c), λex = 687 nm) consists of a broad band from 250 to 500 nm with a maximum at 359.5 nm. This is assigned to 4f–5d transition of Sm2+. As is shown in Fig. 2(d), under the excitation into the 4f55d1 states with the wavelength at 360 nm, the obtained emission spectra of SASO
Sm, the excitation spectrum (Fig. 2(c), λex = 687 nm) consists of a broad band from 250 to 500 nm with a maximum at 359.5 nm. This is assigned to 4f–5d transition of Sm2+. As is shown in Fig. 2(d), under the excitation into the 4f55d1 states with the wavelength at 360 nm, the obtained emission spectra of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm show a broad band emission from 600 to 750 nm overlapped with the f–f transitions of Sm2+. The peak of the broad band emission is 662 nm assigned to f–d transition of Sm2+. The sharp emission bands consists of three groups of lines at 687, 702 and 727 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The dominant line is about 687 nm (5D0 → 7F0 transition of Sm2+ ions), which shows that Sm2+ ions occupy the crystallographic sites without central symmetry in the host.
Sm show a broad band emission from 600 to 750 nm overlapped with the f–f transitions of Sm2+. The peak of the broad band emission is 662 nm assigned to f–d transition of Sm2+. The sharp emission bands consists of three groups of lines at 687, 702 and 727 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The dominant line is about 687 nm (5D0 → 7F0 transition of Sm2+ ions), which shows that Sm2+ ions occupy the crystallographic sites without central symmetry in the host.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% Sm (M = Ba, Sr, Ca) (“*” indicates the strongest peak position of the excitation and emission spectra.)
5% Sm (M = Ba, Sr, Ca) (“*” indicates the strongest peak position of the excitation and emission spectra.)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm show in Fig. 2(e) and (f). The broad excitation band with the peak at 365 nm extends from 250 to 500 nm. And the broad f–d transition emission of Sm2+ ranges from 600 to 750 nm with the peak at 660 nm. The sharp emission bands consists of three groups of lines at 685, 700.5 and 727.5 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The optical properties of M-BASO
Sm show in Fig. 2(e) and (f). The broad excitation band with the peak at 365 nm extends from 250 to 500 nm. And the broad f–d transition emission of Sm2+ ranges from 600 to 750 nm with the peak at 660 nm. The sharp emission bands consists of three groups of lines at 685, 700.5 and 727.5 nm corresponding to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The optical properties of M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm are similar to those of SASO
Sm are similar to those of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm. This is because that the crystal structure of M-BASO is close to that of SASO
Sm. This is because that the crystal structure of M-BASO is close to that of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, thus crystal environment of Sm2+ in M-BASO is near to SASO
Sm, thus crystal environment of Sm2+ in M-BASO is near to SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm. However, compared with that of SASO
Sm. However, compared with that of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, a red-shift of the excitation for M-BASO
Sm, a red-shift of the excitation for M-BASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm can be found and the reason will be discussed in the discussion part.
Sm can be found and the reason will be discussed in the discussion part.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm shows different excitation and emission spectra. The spectra are quite different with the photoluminescence properties of Sm doped SASO and M-BASO samples. Two dominate excitation peaks at 329 nm and 423 nm can be found, which can be assigned to the 4f–5d transition of Sm2+. The emission bands consist of a broad band emission from 550 to 650 nm with the peak at 603 nm. The sharp emission bands consists of three groups of lines at 683.5, 698 and 725.5 nm correspond to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The strongest emission is at 698 (5D0 → 7F1 of Sm2+), which indicates that the Sm2+ ions occupy the crystallographic sites with central symmetry in the host.
Sm shows different excitation and emission spectra. The spectra are quite different with the photoluminescence properties of Sm doped SASO and M-BASO samples. Two dominate excitation peaks at 329 nm and 423 nm can be found, which can be assigned to the 4f–5d transition of Sm2+. The emission bands consist of a broad band emission from 550 to 650 nm with the peak at 603 nm. The sharp emission bands consists of three groups of lines at 683.5, 698 and 725.5 nm correspond to the 5D0 → 7F0, 5D0 → 7F1 and 5D0 → 7F2 transitions of Sm2+, respectively. The strongest emission is at 698 (5D0 → 7F1 of Sm2+), which indicates that the Sm2+ ions occupy the crystallographic sites with central symmetry in the host.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm
Sm

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm is about 2 time stronger than that of SASO
Sm is about 2 time stronger than that of SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, which can be shown in Fig. 4(b) and d. In their crystal structure, as can be shown in Fig. 5(c) and (d), there is one 8f site of Ba with seven different nearest neighbors atoms including two types of Ba–O1, one Ba–O2, one Ba–O3, one Ba–O4, one Ba–O7 and one Ba–O8 bonds. This type of symmetry produces the strong emission of the 5D0–7F0 transition of Sm2+. It has been found that the photoluminescence intensity ratio between 5D0 → 7F2 and 5D0 → 7F1 emission of Eu3+ (I(5D0 → 7F2)/I(5D0 → 7F1)) increases with increasing of the distortion degree of LnO6 or LnO7 polyhedron from that of an ideal octahedron. So, just as the Eu3+, the transition intensity ratio between 5D0–7F0 and 5D0–7F1 emission of Sm2+ (I(5D0 → 7F0)/I(5D0 → 7F1)) is influenced by the distortion degree of LnOn polyhedron from that of an ideal polyhedron.27,28 Their ratio values of I(5D0 → 7F0)/I(5D0 → 7F1) for SASO
Sm, which can be shown in Fig. 4(b) and d. In their crystal structure, as can be shown in Fig. 5(c) and (d), there is one 8f site of Ba with seven different nearest neighbors atoms including two types of Ba–O1, one Ba–O2, one Ba–O3, one Ba–O4, one Ba–O7 and one Ba–O8 bonds. This type of symmetry produces the strong emission of the 5D0–7F0 transition of Sm2+. It has been found that the photoluminescence intensity ratio between 5D0 → 7F2 and 5D0 → 7F1 emission of Eu3+ (I(5D0 → 7F2)/I(5D0 → 7F1)) increases with increasing of the distortion degree of LnO6 or LnO7 polyhedron from that of an ideal octahedron. So, just as the Eu3+, the transition intensity ratio between 5D0–7F0 and 5D0–7F1 emission of Sm2+ (I(5D0 → 7F0)/I(5D0 → 7F1)) is influenced by the distortion degree of LnOn polyhedron from that of an ideal polyhedron.27,28 Their ratio values of I(5D0 → 7F0)/I(5D0 → 7F1) for SASO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm, M-BASO and H-BASO are about 2.21, 3.34 and 0.95, respectively. The distortion degree can be calculated using the standard deviation of environmental factor of the individual bond (EFSD) σ(hei), which can be expressed as below:
Sm, M-BASO and H-BASO are about 2.21, 3.34 and 0.95, respectively. The distortion degree can be calculated using the standard deviation of environmental factor of the individual bond (EFSD) σ(hei), which can be expressed as below: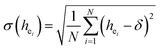
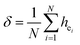
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sm is the lowest.
Sm is the lowest. in the hexagonal crystal H-BASO and monoclinic crystals M-BASO and SASO
in the hexagonal crystal H-BASO and monoclinic crystals M-BASO and SASO
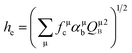
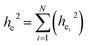
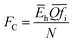
![[Q with combining macron]](https://www.rsc.org/images/entities/i_char_0051_0304.gif) is the average present charge of the anions, and
is the average present charge of the anions, and ![[f with combining macron]](https://www.rsc.org/images/entities/i_char_0066_0304.gif) i is the average bond ionicity between the central ion and the nearest neighbors.
i is the average bond ionicity between the central ion and the nearest neighbors.



