Effect of surfactant on bound water content and extracellular polymers substances distribution in sludge
Chen Hongab,
Yanxiao Sia,
Yi Xing*a,
ZhiQiang Wanga,
Qeng Qiao*ac and
Min Liua
aDepartment of Environmental Engineering, University of Science and Technology Beijing, Xueyuan Road No. 30, Haidian District, Beijing, 100083, P. R. China. E-mail: xingyi@ustb.edu.cn; alanxy3000@gmail.com; Tel: +86 010 62332206
bResearch Centre for Eco-Environmental Sciences, Chinese Academy Science, Shuangqing Road No. 18, Haidian District, Beijing, 100085, P. R. China
cSchool of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
First published on 17th February 2015
Abstract
In this study, the effect of surfactant conditioning on bound water and extracellular polymers substances (EPS) in sludge was investigated. Results showed that by adding dodecyl dimethyl benzyl ammonium chloride (DDBAC), loosely bound EPS (LB-EPS) and slime layer EPS (S-EPS) contents were increased, while tightly bound EPS (TB-EPS) and bound water contents (WB) were reduced. As a result, the dewatering performance of sludge was enhanced. When 75 mg g−1 of DDBAC were added, WB in sludge decreased to 1.64 g g−1, and the water content of dewatered sludge (WC) dropped to 66.61%. EPS partially hydrolyzed into small molecular organics under DDBAC conditioning. Moreover, the quantity and species of organic functional groups in S-EPS were increased obviously. The TB-EPS content accounted for the majority of EPS in sludge, and the protein and polysaccharide in TB-EPS had a significant positive correlation with bound water, which was the main factor affecting WB in sludge.
1 Introduction
In municipal sewage treatment plants, the moisture content of sludge is typically over 95%. Sludge dewatering is the most significant process among sewage treatments in sludge reduction, harmless treatment and reutilization.1,2 However, urban sewage sludge is rich in organic substances, which leads to the formation of colloidal structure in sludge floc particles. These particles are very hydrophilic, as they effortlessly combine with water molecules in different manner. Thus, they make it difficult to remove this part of water from sludge.3 A more detailed classification of water distribution in sludge can be divided into four types of patterns, namely, free water, pore water, adsorbed water, and bound water.4 However, this division has currently no quantitative measurements. Moisture is usually divided into free and bound types of water through usual quantitative methods.5 Free water is not restrained by sludge floc bondage, but it can be separated from sludge through concentration or mechanical dewatering. Moreover, bound water has a strong bond with sludge flocs. It is specifically tightly restrained in sludge and it is difficult to remove through mechanical force.6The presence of bound water has a close relationship with extracellular polymers substances (EPS) in sludge. EPS is an insoluble organic matter attached to the surface of the sludge bacterial cell, which is the third largest composition in addition to bacterial cells and water.7 EPS accounts for 50% to 90% of total organic matter in activated sludge, and it can stabilize sludge floc structures by connecting microbial cells and other substances.8 EPS binds water to the solid surfaces or capture water inside the cells or flocs because of its strong water binding capacity.9–11 Thus, EPS is a key factor affecting the stability and dewatering of sludge.12 The main components of EPS are proteins and polysaccharides, which account for 70% to 80% of total EPS.13,14 According to the combination degree between organic matter and sludge flocs, EPS can be divided into slime layer EPS (S-EPS), loosely bound EPS (LB-EPS), and tightly bound EPS (TB-EPS).15 Jin et al.6 argued that the polymeric component such as proteins and carbohydrates significantly contribute to improve the water binding ability of sludge flocs.
In studies of sludge dewatering, numerous researchers concluded that adding surfactants could improve sludge dewatering performance.16–20 Chen et al.16 reported that in addition to the enhancement of the ability to demolish the sludge structure, the dissolution of suspended solids and digestion of extracellular components could be enhanced by the introduction of a surfactant (lauryl betaine), compared to traditional sludge conditioner (FeCl3, CaO). Furthermore, the protein and carbohydrate concentrations in the sludge liquid increased, and the dewatering and filtering performance were also enhanced. Yuan et al.20 used electrolysis and surfactant joint conditioning sludge in their study. The results demonstrated that adding surfactant helped to increase the concentration of S-EPS, reduce viscosity and zeta potential of the sludge, and improve the sewage sludge dewatering performance. Current studies focus on the relationship between the S-EPS content and the sludge dewatering performance under surfactant conditioning. The effect of surfactant on LB-EPS and TB-EPS, and the relation between EPS layers (S-EPS, LB-EPS, and TB-EPS) and bound water under surfactant conditioning is not well known yet.
The effect of surfactant (dodecyl dimethyl benzyl ammonium chloride, DDBAC) conditioning on EPS and bound water in sludge was investigated in this study. The impact of surfactant on dewatered sludge water content, bound water content, the content of protein and polysaccharide in different EPS layers, and the relation between bound water content and EPS distribution were analyzed. Fourier transform infrared spectroscopy (FTIR) and high-performance liquid chromatography (HPLC) were used to test sludge supernatant to demonstrate the changes in organic matter in quantity and variety. The aim is to provide a more comprehensive study on the sludge dewatering mechanism under surfactant conditioning.
2 Materials and methods
2.1 Materials
The sludge used in this study was collected from the sludge concentration tank in Xiaohongmen sewage treatment plant, Beijing. It was dewatered to 95% in terms of water content before being employed as test sample. The properties of the sludge before conditioning in this study are shown in Table 1. All the experiments were completed within 48 h and the sludge was placed in a freezer at 4 °C beforehand.| Parameters | Units | Value | |
|---|---|---|---|
| TSS | mg L−1 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952.55 ± 244.78 952.55 ± 244.78 |
|
| VSS | mg L−1 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843.52 ± 1644.26 843.52 ± 1644.26 |
|
| pH value | — | 7.19 ± 0.12 | |
| Water content | % | 95.07 ± 0.15 | |
| Bound water | g g−1 | 3.24 ± 0.26 | |
| S-EPS | Protein | mg L−1 | 363.31 ± 18.23 |
| Polysaccharide | mg L−1 | 72.68 ± 6.74 | |
| LB-EPS | Protein | mg L−1 | 143.16 ± 7.57 |
| Polysaccharide | mg L−1 | 29.06 ± 3.34 | |
| TB-EPS | Protein | mg L−1 | 3045.02 ± 150.46 |
| Polysaccharide | mg L−1 | 602.54 ± 33.52 | |
Sludge conditioner is a cationic surfactant (dodecyl dimethyl benzyl ammonium chloride, DDBAC), with a chemical formula C21H38NCl and has a relative molecular mass of 340.00.
2.2 Experimental methods
 | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). The supernatant was filtered through a 0.45 μm membrane, in which the residual organics in the filtrate were regarded as TB-EPS.
000 rpm). The supernatant was filtered through a 0.45 μm membrane, in which the residual organics in the filtrate were regarded as TB-EPS.The Folin-phenol (Lowry) method was employed to determine the extracellular protein content, and an anthrone colorimetric method was used to measure the polysaccharide content.23,24
Chromatography was employed under the following conditions: the mobile phase was 90% CH3CN–0.10 mol L−1 CH3COONH4 solution (v/v) filtered through a 0.45 μm membrane, and then degassed by ultrasonication for 10 min. The flow rate was 0.5 mL min−1, column temperature was 25 ± 1 °C, measurement wavelength was 262 nm, injection volume was 20 μL, and the high peaks were quantified via the external standard method.
② Organic matter hydrolysis in sludge supernatant was analyzed by liquid chromatography. HPLC was applied to determine the content of small molecular organic acids (formic acid, acetic acid, and propionic acid). Liquid chromatography could measure the organic compounds hydrolysis.
Chromatographies were performed under the following conditions: the mobile phase was 7% CH3OH–0.20 mol L−1 KH2PO4 (pH = 4.0) buffer solution (v/v) filtered through a 0.45 μm membrane, and then degassed by ultrasonication for 10 min. The flow rate was 0.5 mL min−1, column temperature was 40 ± 1 °C, measurement wavelength was 215 nm, the injection volume was 20 μL, and the high peaks were quantified via the external standard method.
3 Results and discussion
3.1 Changes of WC and composition in dewatered sludge after conditioning with surfactant DDBAC
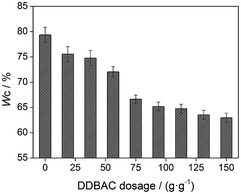
As shown in Fig. 1, the dewatering performance of sludge under conditioning significantly improved when the surfactant (DDBAC) dosage was increased. WC decreased from 79.36% (DDBAC dosage was 0 mg g−1, mg g−1 indicated DDBAC dosage per gram of dry sludge in the sample, similarly hereinafter) to 62.95% (DDBAC dosage was 150 mg g−1), indicating a 16.41% decline. When the DDBAC dosage increased from 56.25 mg g−1 to 75 mg g−1, WC significantly decreased from 72.05% to 66.61%, representing a 5.44% decline. Moreover, when the DDBAC dosage was greater than 75.00 mg g−1, the downward trend of WC slowed down. These results were in agreement with Chen et al.16 and Yuan et al.,20 who observed similar results in sludge dewatering performance, improving significantly under surfactants conditions.The decreasing trend of WC was due to the reduction of WB. WB was held firmly in the floc matrix, bound to the sludge or trapped between sludge particles and could not be removed by mechanical methods. This was considered to be the limit of mechanical dewatering. According to Colin and Gazbar,25 WB could be used to directly measure the difficulty degree of mechanical dewatering. More bound water resulted in more difficult mechanical dewatering, whereas less bound water resulted in easier mechanical dewatering. The WB under conditioning with surfactant DDBAC is presented in Fig. 2(a). Addition of DDBAC could significantly reduce WB. When the DDBAC dosage increased from 0 mg g−1 to 75 mg g−1, WB quickly decreased from 3.24 g g−1 (g g−1 indicated WB per gram of dry sludge in sample, similarly hereinafter) to 1.64 g g−1, indicating a drop of 49.38%. When the DDBAC dosage was continuously increased, the decreasing trend of WB slowed down. WB and WC were ramping down in a similar trend (Fig. 1 and 2(a)). These results indicated that the introduction of DDBAC was beneficial to release bound water from sludge, and then caused the decrease of WC. Eventually, when the DDBAC dosage was 150 mg g−1, WB reduced to 1.36 g g−1, representing a 58.02% decline. These findings were in agreement with a research reported by Wang et al.26 who observed that the surfactant effectively released bound water from sludge, due to its superior surface activity and strong adsorption/bridge capacities with sludge.
 | ||
| Fig. 2 WB and composition distribution of dewatered sludge: (a) WB; (b) composition in dewatered sludge. | ||
Approximately 42% of bound water in sludge could not be released. A reasonable explanation was that this part of bound water combined with sludge flocs through a considerably strong chemically/physically bond, and was not easily influenced by DDBAC or released into the liquid phase.
Relative proportions of solid, bound and free water in dewatered sludge obviously changed after conditioning with DDBAC, as shown in Fig. 2(b). After conditioning with DDBAC, the proportion of bound water in sludge cake significantly decreases from 66.87% (unconditioned sludge cake) to 49.69% (DDBAC dosage was 150 mg g−1), and the proportion of solid increases from 20.64% to 36.58%. WB was considered as a critical factor for the sludge dewatering performance. The surfactant DDBAC conditioning did not only significantly reduce WB and WC, but also increased the total solid proportion in the dewatered sludge. The proportion of free water in the sludge cake changed slightly and remained between 11% and 13%, which indicated the presence of free water in the sludge cake. This happened because the mechanical strength in the dehydration process was not enough to remove the free water. The removal rate of free water by mechanical force depended on the mechanical dewatering device and had no correlation with sludge conditioning.
3.2. Effect of EPS on bound water under the surfactant DDBAC conditions
 | ||
| Fig. 3 Changes of EPS in sludge: (a) distributions and changes of protein in EPS layers; (b) distributions and changes of polysaccharide in EPS layers. | ||
After conditioning with surfactant DDBAC, protein and polysaccharide contents in EPS layers obviously changed. These contents in TB-EPS were decreased by adding DDBAC. When the DDBAC dosage was 75 mg g−1, the content of protein in TB-EPS droped to 1737 mg L−1, when it was 3045 mg L−1 beforehand, and the polysaccharide content changed from 602 mg L−1 to 421 mg L−1, indicating a decrease of 42.96% and 30.07%, respectively. When the DDBAC dosage continued to increase, the decreasing rate of protein and polysaccharide contents in the TB-EPS slowed down. On the contrary, the introduction of DDBAC caused these contents to increase in LB-EPS and S-EPS. When the DDBAC dosage was 75 mg g−1, the protein content in LB-EPS increased from 143 mg L−1 to 374 mg L−1, and the polysaccharide content increased from 29 mg L−1 to 65 mg L−1, accounting for increases of 161.54% and 124.14%, respectively. At the same time, the protein and polysaccharide contents in S-EPS increased from 363 mg L−1 to 561 mg L−1 and from 73 mg L−1 to 120 mg L−1, accounting for increases of 54.55% and 64.38%, respectively. These results were in accordance with Chen et al.16 and Yuan et al. studies.30 They observed that the EPS content of sludge supernatant (corresponding to S-EPS) increased with the increasing surfactant. When the surfactant DDBAC dosage was more than 75 mg g−1, it had a less significant effect on protein and polysaccharide contents in the LB-EPS and S-EPS.
The surfactant DDBAC can help in dispersing sludge folcs and dissolving organic matters. The binding effect of sludge flocs on the TB-EPS and LB-EPS is weakened by adding the surfactant. Therefore, parts of TB-EPS could turn into LB-EPS or S-EPS, and LB-EPS could turn into S-EPS. Moreover, insoluble EPS (TB-EPS and LB-EPS), which had high hydration ability, can be turned into soluble EPS (S-EPS). Soluble EPS left the sludge flocs surface and easily entered the sludge liquid phase. Thus, bound water previously combined with EPS was released and turned to free water, and therefore, the sludge dewatering performance could be improved. This process was in accordance with the decrease in TB-EPS content, increase in LB-EPS and S-EPS contents, and decline of WB under DDBAC conditioning (Fig. 2 and 3). Similar conclusions were reached by S. Kavitha32 and Huang,33 whose results indicated that surfactants could not only efficiently disperse sludge flocs, but are also beneficial to solubilize organic matters.
The adsorption amounts of DDBAC in sludge are illustrated by Fig. 4. The adsorption process between EPS and surfactant was influenced by both electrostatic effect and hydrophobic effect.34 Under these two effects, EPS was desquamated from sludge flocs, and DDBAC got into the sludge liquid-phase with peeled LB-/TB-EPS. The adsorption amounts of DDBAC in sludge first increased and then decreased. When the DDBAC dosage was 56.25 mg g−1, the maximum adsorption amounts of DDBAC could be obtained was 42.82 mg g−1. Then, the adsorption amounts of DDBAC decreased to 36.13 mg g−1 when the DDBAC dosage increased to 75 mg g−1. With the continuous increase in DDBAC dosage, the DDBAC adsorption amounts plateaued, and the alterations of WB and EPS became small, indicating that the conditioning effect of DDBAC on sludge weakened (Fig. 2 and 3). Therefore, 75 mg g−1 was considered to be the optimal dosage of DDBAC.
A correlation analysis demonstrated that S-EPS, LB-EPS, and TB-EPS had strong correlations with bound water. The increase in protein and polysaccharide contents in the S-EPS and LB-EPS and their decrease in the TB-EPS could cause a drop of WB. TB-EPS accounts for over 85% of total EPS in the original sludge. The decrease in protein and polysaccharide contents in TB-EPS led to the increase in EPS contents in the LB-EPS and S-EPS under DDBAC conditioning (Fig. 3). Thus, it can be inferred that the bound water trapped in the TB-EPS and the TB-EPS content was the main factor that influenced WB. These findings were similar to a research reported by Dursun,35 who observed that EPS could result in a fairly tenacious retention of water within the sludge. Song et al.36 also draw the conclusion that high contents of EPS, especially insoluble EPS, were responsible for the relatively higher WB. Yuan et al.30 observed a similar phenomenon, showing that TB-EPS may be responsible for the water retention of sludge and could highly influence the sludge dewatering and drying.
3.3. Change of organic functional groups in S-EPS
The FTIR spectra of S-EPS extracted from sludge under conditioning with different DDBAC dosages are shown in Fig. 6. S-EPS in the original sludge exhibit several obvious absorption peaks at 3394 cm−1, 2926 cm−1, 1636 cm−1, 1428 cm−1, and 1108 cm−1. Absorption maxima near 3340 cm−1 (H-bound OH in polysaccharides), 2950 cm−1 (aliphatic C–H stretching), 1600 cm−1 to 1660 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in amides, protein peptide bond), 1420 cm−1 (C
O in amides, protein peptide bond), 1420 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxyl groups), and 1090 cm−1 (C–O–C stretching of polysaccharides) had been reported in other studies.37–39 In all the spectra we observed that the broad band at 3394 cm−1 confirmed the presence of abundant OH groups, which would indicate that S-EPS may contain polysaccharides.39 The absorption band in the region of 2926 cm−1 corresponded to the stretching vibrations of aliphatic C–H bonds that show the existence of lipids. The presence of amides I was shown by a strong absorbance at 1636 cm−1, corresponding to protein in the S-EPS.37 The weak absorption band at 1428 cm−1 indicated the existence of carboxyl groups because of the stretching vibration of C
O in carboxyl groups), and 1090 cm−1 (C–O–C stretching of polysaccharides) had been reported in other studies.37–39 In all the spectra we observed that the broad band at 3394 cm−1 confirmed the presence of abundant OH groups, which would indicate that S-EPS may contain polysaccharides.39 The absorption band in the region of 2926 cm−1 corresponded to the stretching vibrations of aliphatic C–H bonds that show the existence of lipids. The presence of amides I was shown by a strong absorbance at 1636 cm−1, corresponding to protein in the S-EPS.37 The weak absorption band at 1428 cm−1 indicated the existence of carboxyl groups because of the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.37 The strong absorption band at 1088 cm−1 was due to the stretching of C–O–C, which demonstrated the existence of polysaccharide in S-EPS.38
O.37 The strong absorption band at 1088 cm−1 was due to the stretching of C–O–C, which demonstrated the existence of polysaccharide in S-EPS.38
Peak intensities at 3394 cm−1, 1636 cm−1, 1428 cm−1, 1108 cm−1 significantly enhanced after DDBAC conditioning. The increase of amides and polysaccharides (at 3394 cm−1, 1636 cm−1, and 1108 cm−1) indicated that a large amount of protein and polysaccharides turned from insoluble into dissolved state under DDBAC conditioning, and could be found in the sludge liquid phase. This observation was in accordance with the decrease in TB-EPS content and the increase in LB-EPS in earlier results. A significant enhancement in peak intensity at 1428 cm−1 indicated that the most possible reason of carboxyl increment was that the hydrolysis of TB-EPS and LB-EPS generated a certain amount of carboxylic acid, carboxylic acid salts, and other small organic molecules. When the DDBAC dosage was more than 37.5 mg g−1, two new adsorption bands were observed at 1511 cm−1 and 1348 cm−1. According to previous studies, the absorption band at 1511 cm−1 was likely the consequence of the vibrations of amides II and/or of N–H bonds.40,41 It indicated that more new types of insoluble protein in TB-EPS and LB-EPS turned into dissolved ones in S-EPS, and bound water was released in a high degree. A weak band was observed at 1348 cm−1, which was related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic acid and H-bound OH in alcohols and phenols. This finding indicated that EPS had been hydrolyzed in some degree.
O in carboxylic acid and H-bound OH in alcohols and phenols. This finding indicated that EPS had been hydrolyzed in some degree.
3.4. Analysis of EPS hydrolysis under the conditioning of surfactant DDBAC
The liquid chromatogram of organics in the sludge supernatant is shown in Fig. 7(a), and the changes in short-chain fatty acids (formic acid, acetic acid, and propionic acid in this study) contents are reported in Fig. 7(b). The sequence of chromatography peaks in the reversed-phase high-performance liquid chromatography was relevant to the molecular weight and polarity of the organics. Generally, the peak of organics that had small molecular weight and strong polarity appear earlier than those that have a significantly larger molecular weight, high polymerization degree, and strong polarity. Peak intensity was positively in accordance with the amount of organic content. In this study, the retention times of formic acid, acetic acid, and propionic acid peak were 5.73 min, 8.73 min, and 18.32 min, respectively. According to previous studies, these organics that had retention times between 5 min and 22.5 min were small molecular organics. In Fig. 7(a), the number and intensity of chromatographic peaks significantly increased after the DDBAC conditioning, which demonstrated an increase in the species and contents of small molecular organics. Hydrolysis was demonstrated by short-chain fatty acids concentration in the sludge supernatant. The three organic acids, particularly formic acid and acetic acid, significantly increased along with the increase in DDBAC dosages, which indicated that the DDBAC could improve the hydrolysis of organics in sludge (Fig. 7(b)). Luo et al.42 observed that surfactants can be used to enhance the hydrolysis of sludge and to increase the short-chain fatty acids concentration. Furthermore, Huang et al.33 pointed out that the high surface activity of the surfactant may be the fundamental reason of the enhancement of organic matters solubilization and hydrolysis, which was beneficial for small molecular organics production. Chen et al.43 raised another reason, he reported that the surfactant caused changes in protein structure and benefited protein hydrolysis. Therefore, the surfactant DDBAC was not only able to dissolve and desquamate the combined extracellular polymeric substance in sludge flocs, but also boost macromolecular organic compounds, such as protein and polysaccharide in EPS, hydrolyzing into small molecular organics, which had a beneficial effect on the release of bound water in sludge. | ||
| Fig. 7 EPS hydrolysis under the conditioning of DDBAC: (a) liquid chromatogram of organics in sludge supernatant, (b) organic acid contents in sludge supernatant. | ||
4 Conclusions
Surfactant DDBAC could effectively reduce WB and improve the dewatering performance of sludge. When DDBAC dosage was 75 mg g−1, WB dropped from 3.24 g g−1 to 1.64 g g−1, a 49.38% decrease was observed. Moreover, WC decreased to 66.61%.The binding effect of sludge flocs on TB-EPS and LB-EPS layers was weakened by adding DDBAC, which enhanced the solubility of TB-/LB-EPS. Therefore, parts of TB-EPS turned into LB-EPS and S-EPS, and parts of LB-EPS turned into S-EPS.
The quantities of organic functional groups and small molecular organics increased obviously in the sludge supernatant, which showed that EPS was hydrolyzed by DDBAC as well.
The amount of TB-EPS was more than 85% in total EPS in sludge. Protein and polysaccharide contents in TB-EPS had significant positive correlation with WB. TB-EPS were considered to be the main factor influencing WB.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (51104009), Beijing Nova Program (Z111106054511043), Beijing outstanding talent nurture and funding scheme (2012D009006000003), Fundamental Research Funds for the Central Universities (FRF-TP-12-011B), and Kunming Science and Technology Program (2012-02-09-A-G-02-0001).Notes and references
- M. Citeau, O. Larue and E. Vorobiev, Water Res., 2006, 45, 2167–2180 CrossRef PubMed.
- M. Tokumura, M. Sekine, M. Yoshinari, H. T. Znad and Y. Kawase, Process Biochem., 2007, 42, 627–633 CrossRef CAS PubMed.
- X. Li and S. Yang, Water Res., 2007, 41, 1022–1030 CrossRef CAS PubMed.
- P. A. Vesilind and C. J. Martel, J. Environ. Eng., 1990, 116, 854–862 CrossRef CAS.
- P. A. Vesilind, Water Environ. Res., 1994, 66, 4–11 CrossRef CAS.
- B. Jin, B. M. Wilén and P. Lant, Chem. Eng. J., 2004, 98, 115–126 CrossRef CAS PubMed.
- G. H. Yu, P. J. He, L. M. Shao and P. P. He, Environ. Sci. Technol., 2008, 42, 7944–7949 CrossRef CAS.
- V. Urbain, J. Block and J. Manem, Water Res., 1993, 27, 829–838 CrossRef CAS.
- K. Keiding, L. Wybrandt and P. Nielsen, Water Sci. Technol., 2001, 43, 17–23 CAS.
- D. Mowla, H. N. Tran and D. G. Allen, Biomass Bioenergy, 2013, 58, 365–378 CrossRef CAS PubMed.
- A. Ding, F. Qu, H. Liang, S. Guo, Y. Ren, G. Xu and G. Li, RSC Adv., 2014, 47, 24762–24768 RSC.
- X. Yin, P. Han, X. Lu and Y. Wang, Ultrason. Sonochem., 2004, 11, 337–348 CAS.
- M. F. Dignac, V. Urbain, D. Rybacki, A. Bruchet, D. Snidaro and P. Scribe, Water Sci. Technol., 1998, 38, 45–53 CrossRef CAS.
- J. I. Houghton and T. Stephenson, Water Res., 2002, 36, 3620–3628 CrossRef CAS.
- T. L. Poxon and J. L. Darby, Water Res., 1997, 31, 749–758 CrossRef CAS.
- Y. G. Chen, Y. S. Chen and G. Gu, Chem. Eng. J., 2004, 99, 137–143 CrossRef CAS PubMed.
- S. Chitikel and S. K. Dentel, Water Environ. Res., 1998, 70, 1062–1069 CrossRef.
- C. Chu, D. Lee and C. Huang, J. Colloid Interface Sci., 1998, 206, 181–188 CrossRef CAS PubMed.
- C. Huang and G. Fu, Water Sci. Technol., 2000, 41, 17–22 Search PubMed.
- H. Yuan, N. Zhu and F. Song, Bioresour. Technol., 2011, 102, 2308–2315 CrossRef CAS PubMed.
- J. Vaxelaire and P. Cézac, Water Res., 2004, 38, 2215–2230 CrossRef CAS PubMed.
- G. H. Yu, P. J. He, L. M. Shao and D. J. Lee, Appl. Microbiol. Biotechnol., 2007, 77, 605–612 CrossRef CAS PubMed.
- O. Classics Lowry, N. Rosebrough, A. Farr and R. Randall, J. Biol. Chem., 1951, 193, 265–275 Search PubMed.
- P. Riesz, D. Berdahl and C. Christman, Environ. Health Perspect., 1985, 64, 233–252 CrossRef CAS.
- F. Colin and S. Gazbar, Water Res., 1995, 29, 2000–2005 CrossRef CAS.
- L. F. Wang, D. Q. He, Z. H. Tong, W. W. Li and H. Q. Yu, Biochem. Eng. J., 2014, 91, 174–178 CrossRef CAS PubMed.
- X. Feng, J. Deng, H. Lei, T. Bai, Q. Fan and Z. Li, Bioresour. Technol., 2009, 100, 1074–1081 CrossRef CAS PubMed.
- Y. Liu and H. H. Fang, Crit. Rev. Environ. Sci. Technol., 2003, 33, 237–273 CrossRef CAS.
- B. M. Wilén, B. Jin and P. Lant, Water Res., 2003, 37, 3632–3645 CrossRef.
- D. Yuan and Y. Wang, Biochem. Eng. J., 2013, 77, 208–213 CrossRef CAS PubMed.
- H. Wang, H. Deng, L. Ma and L. Ge, Carbohydr. Polym., 2013, 92, 510–515 CrossRef CAS PubMed.
- S. Kavitha, C. Jayashree, S. Adish Kumar, I. T. Yeom and J. Rajesh Banu, Bioresour. Technol., 2014, 168, 159–166 CrossRef CAS PubMed.
- X. F. Huang, C. M. Shen, J. Liu and L. J. Lu, Chem. Eng. J., 2015, 264, 280–290 CrossRef CAS PubMed.
- W. J. Lv, Y. F. Hu, B. C. Zhan, Z. H. Liu, Y. Z. Shang, H. Y. Wang and H. L. Liu, Acta Phys.-Chim. Sin., 2014, 30, 811–820 Search PubMed.
- D. Dursun, Ph.D. thesis, University of Delaware, 2007.
- Y. W. Song, G. Y. Zheng, M. B. Huo, B. W. Zhao and L. X. Zhou, Environ. Technol., 2014, 35, 2538–2545 CrossRef CAS PubMed.
- A. R. Badireddy, S. Chellam, P. L. Gassman, M. H. Engelhard, A. S. Lea and K. M. Rosso, Water Res., 2010, 44, 4505–4516 CrossRef CAS PubMed.
- L. El Fels, M. Zamama, A. El Asli and M. Hafidi, Int. Biodeterior. Biodegrad., 2014, 87, 128–137 CrossRef CAS PubMed.
- L. Zhu, H. Y. Qi, M. L. Lv, Y. Kong, Y. W. Yu and X. Y. Xu, Bioresour. Technol., 2012, 124, 455–459 CrossRef CAS PubMed.
- A. Barth and C. Zscherp, Q. Rev. Biophys., 2002, 35, 369–430 CrossRef CAS.
- M. Mecozzi and E. Pietrantonio, Mar. Chem., 2006, 101, 27–39 CrossRef CAS PubMed.
- K. Luo, Q. Yang, J. Yu, X. M. Li, G. J. Yang, B. X. Xie, F. Yang, W. Zheng and G. M. Zeng, Bioresour. Technol., 2011, 102, 7103–7110 CrossRef CAS PubMed.
- Y. G. Chen, K. Liu, Y. L. Su, X. Zheng and Q. Wang, Bioresour. Technol., 2013, 140, 97–102 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |