Open Access Article
Open Access ArticleUnexpected rearrangement during the gas-phase dehalogenation approach to benzodithiophenes†
R. Alan Aitken* and
Adebayo O. Oyewale‡
EaStCHEM School of Chemistry, University of St Andrews, North Haugh, St Andrews, Fife KY16 9ST, UK. E-mail: raa@st-and.ac.uk; Fax: +44 (0)1334 463808; Tel: +44 (0)1334 463865
First published on 9th February 2015
Abstract
A range of halogenated methylthiophenes undergo dehalogenative condensation upon FVP over magnesium or calcium to give products including benzodithiophenes; in some cases this involves a novel rearrangement by equilibration of alkenylthienyl radicals via thiophene-fused cyclopropylmethyl intermediates.
Some time ago we described the use of flash vacuum pyrolysis (FVP) over a bed of freshly sublimed magnesium as an effective and general method for dehalogenative coupling of benzyl halides, mono and dihaloalkanes and related compounds, with various pieces of evidence favouring the intermediacy of surface-adsorbed organometallic species rather than free gas-phase radicals.1,2 Although the six possible benzodithiophenes were prepared by conventional multi-step synthesis many years ago,3 related compounds such as a dialkylbenzodithiophene4 and naphthodithiophenes5,6 have been of recent interest for their electronic properties and this inspired us to apply the gas phase dehalogenation method to their synthesis.
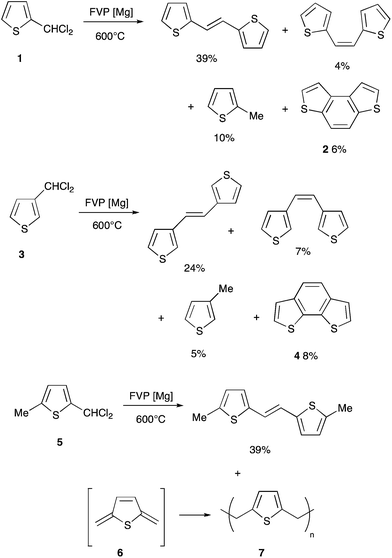
The required dichloromethylthiophenes were prepared by reaction of the corresponding aldehydes with PCl5 and in common with previous workers,7 we found them to be highly unstable so they were used immediately for the pyrolysis studies. Their 13C NMR spectra, not previously reported, all showed a distinctive signal at 62–68 ppm for the CHCl2 carbon. We previously described briefly the reaction of a range of substituted benzylidene chlorides ArCHCl2 over magnesium under FVP conditions which resulted in complete dehalogenation to give mainly the corresponding stilbenes,1 and this pattern was repeated in the thiophene series. Thus, FVP of 2-dichloromethylthiophene 1 at 600 °C through glass wool coated with freshly sublimed magnesium gave mainly the (E)- and (Z)-dithienylethenes along with a little 2-methylthiophene and the cyclisation product benzo[1,2-b:4,3-b′]dithiophene 2 identified by comparison with an authentic sample3 (Scheme 1). Likewise the isomeric 3-dichloromethylthiophene 3 gave the (E)- and (Z)-alkenes, the methylthiophene and benzo[2,1-b:3,4-b′]dithiophene 4. The methyl compound 5 gave mainly the known8 crystalline (E)-alkene but this was accompanied by an insoluble polymer assumed to be poly(2,5-thienyleneethylene) 7 formed from the quinonoid dimethylene compound 6. In the previous work,1 cyclisation was promoted by an ortho halogen substituent and 2-chlorobenzylidene chloride gave phenanthrene in 47% yield. It was therefore expected that benzodithiophene formation would be increased with thiophenes bearing adjacent dichloromethyl and halogen substituents. The two new compounds 8 and 15 were therefore prepared from the corresponding aldehydes9,10 by reaction with PCl5 and subjected to FVP over magnesium.
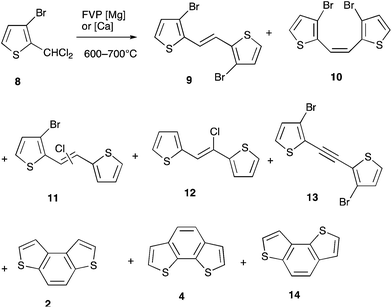
With compound 8 at 600 °C there was only partial reaction to give mainly products 9–13 still containing halogen (Scheme 2, Table 1) so the temperature was increased to 650 °C. This had the desired effect in reducing halogen-containing products and increasing the benzodithiophene yield. However, most surprisingly, not only the expected isomer 2, but also 4 and 14 were observed. The volatility of magnesium places an upper limit of around 650 °C on the temperature of operation, so we substituted it with calcium for which 700 °C is easily accessible and, under these conditions, there was even more complete dehalogenation to give as the main product a mixture of the three isomeric benzodithiophenes 2, 4 and 14.
| Starting material | Metal | Temperature °C | Products (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9/16 | 10/17 | 11 | 12 | 13/18 | 19 | 20 | 2 | 4 | 14 | |||
| 8 | Mg | 600 | 25 | 4 | 13 | 6 | 3 | Trace | ||||
| 8 | Mg | 650 | 14 | 4 | 10 | 12 | Trace | 8 | 10 | 6 | ||
| 8 | Ca | 700 | Trace | Trace | 8 | 16 | 16 | 10 | ||||
| 15 | Mg | 600 | 28 | 10 | 6 | 18 | 10 | 2 | 2 | |||
| 15 | Mg | 650 | 24 | 8 | 2 | 6 | ||||||
| 15 | Ca | 700 | 21 | 3 | 12 | 9 | 3 | 3 | 9 | |||
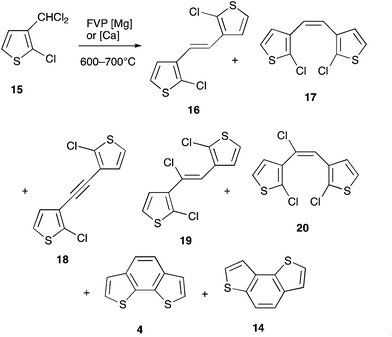
With the pseudoisomeric system 15 similar results were obtained: at 600 °C the main products were 16–20 along with not only the expected benzodithiophene 4 but also the isomer 14. Either moving to 650 °C with magnesium or 700 °C with calcium served to increase the yield of the latter two compounds with the rearranged product 14 actually predominating over 4 (Scheme 3, Table 1).
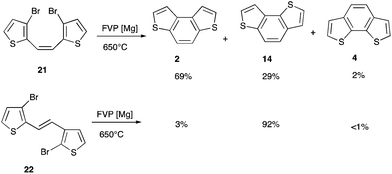
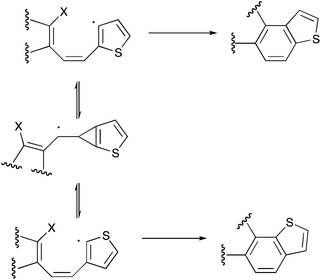
It therefore appears that, under the conditions used, an unusual isomerisation process is occurring. Authentic samples of both 23 and 1411 were prepared and resubjected to the pyrolysis conditions but were recovered completely unchanged. The key evidence as to the mechanism of the rearrangement was obtained by synthesis3 of the expected intermediates 21 and 22 formed by dehalogenative coupling of the dichloromethyl groups but with the ring halogens still in place. Subjecting either of these compounds to FVP over magnesium at 650 °C gave a mixture of the three isomeric benzodithiophenes 2, 4, and 14 (Scheme 4). In both cases the main product was the one expected without rearrangement but the formation of the other isomers and particularly a significant amount of 14 from 21 as well as from 22 supports the occurrence of the rearrangement process at this stage in the dehalogenation.
In view of all the evidence we propose that this occurs as shown in Scheme 5 by way of a cyclopropenylmethyl radical (or its metal-adsorbed equivalent) which can be formed either from the 2-alkenyl-3-thienyl or 3-alkenyl-2-thienyl radicals and this provides a mechanism for interconversion between them leading to the two different modes of thiophene ring fusion. Further applications of FVP over reactive metals will be reported shortly.
Notes and references
- R. A. Aitken, P. K. G. Hodgson, A. O. Oyewale and J. J. Morrison, Chem. Commun., 1997, 1163–1164 RSC.
- R. A. Aitken, P. K. G. Hodgson, J. J. Morrison and A. O. Oyewale, J. Chem. Soc., Perkin Trans. 1, 2002, 402–415 RSC.
- S. Gronowitz and T. Dahlgren, Chem. Scr., 1977, 12, 57–67 CAS.
- T. M. Pappenfus, D. T. Seidenkranz and E. W. Reinheimer, Heterocycles, 2012, 85, 355–364 CrossRef CAS.
- R. Umeda, H. Fukuda, K. Miki, S. M. A. Rahman, M. Sonoda and Y. Tobe, C. R. Chimie, 2009, 12, 378–384 CrossRef CAS PubMed.
- S.-W. Cheng, D.-Y. Chiou, Y.-Y. Lai, R.-H. Yu, C.-H. Lee and Y.-J. Cheng, Org. Lett., 2013, 15, 5338–5341 CrossRef CAS PubMed.
- M. S. Baird and I. Bruce, J. Chem. Res., Synop., 1990, 134–135 (J. Chem. Res., Miniprint, 1990, 946–965) CAS.
- R. V. Hoffman, G. G. Orphanides and H. Shechter, J. Am. Chem. Soc., 1978, 100, 7927–7933 CrossRef CAS.
- S. Gronowitz, P. Moses, A.-B. Hörnfeldt and R. Håkansson, Ark. Kemi, 1961, 17, 165–177 CAS.
- B. Yom-Tov and S. Gronowitz, Chem. Scr., 1973, 3, 165–170 CAS.
- R. M. Kellogg, M. B. Groen and H. Wynberg, J. Org. Chem., 1967, 32, 3093–3100 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details for synthetic and pyrolytic studies. See DOI: 10.1039/c4ra15004j |
| ‡ Current address: Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria, E-mail: E-mail: aooyewale@abu.edu.ng. |
| This journal is © The Royal Society of Chemistry 2015 |