Physicochemical characteristics of attached biofilm on granular activated carbon for thermophilic biohydrogen production
Nabilah Aminah
Lutpi
ab,
Jamaliah Md
Jahim
*ac,
Tabassum
Mumtaz
c,
Peer Mohamed
Abdul
a and
Mohd Tusirin
Mohd Nor
d
aDepartment of Chemical and Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia. E-mail: jamal@eng.ukm.my; Fax: +60-03-89216148; Tel: +60-03-89216427/+60-03-89216412
bSchool of Environmental Engineering, Universiti Malaysia Perlis, 01000 Kangar, Perlis, Malaysia
cFuel Cell Institute, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia
dChair for Sustainable Development Zero Waste Technology, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia
First published on 4th February 2015
Abstract
In this study, thermophilic biohydrogen production by a mixed culture, obtained from a continuous acidogenic reactor treating palm oil mill effluent, was improved by using granular activated carbon (GAC) as the support material. Batch experiments were carried out at 60 °C by feeding the anaerobic sludge bacteria with a sucrose-containing synthetic medium at an initial pH of 5.5 under anoxic conditions. The physico-chemical characteristics of the attached biofilm were evaluated after extraction of the extracellular polymeric substances (EPSs) of the biofilm using the formaldehyde–NaOH method. The main component of the biofilm was protein (60%), while the carbohydrate content accounted for 40% of the EPS. Two major absorption bands at approximately 3400 cm−1 and 1650 cm−1, characteristics of the stretching vibrations of hydroxyl and amino groups, respectively, were identified in the FT-IR spectra, confirming the composition of the EPS. Observations using scanning electron microscopy (SEM) illustrated the attachment of rod-shaped bacterial cells on the GAC at 60 °C. A maximum hydrogen production rate of 4.3 mmol L−1 h−1 and a hydrogen yield of 5.6 mol H2 per mol sucrose were obtained from this attached biofilm system. The major soluble metabolites of fermentation were acetic acid and butyric acid. The results showed that the granular activated carbon enhanced the biohydrogen production by stabilizing the pH and microbial metabolites and therefore could be used as a support material for fermentative hydrogen production under thermophilic conditions on a large scale.
1. Introduction
Hydrogen is a clean renewable energy with great potential as a substitute for the depletion of limited fossil fuels. There are various ways to produce hydrogen. For example, anaerobic or fermentative hydrogen production is one of the most promising ways to stabilise waste organics and convert biomass into hydrogen using specific anaerobic bacteria. This process can fulfil sustainable development as it only requires little energy input, utilises waste organics as substrates, and produces hydrogen, which is a noncarbonaceous energy carrier.Various microorganisms have been exploited to produce hydrogen, either in suspended or attached growth systems. Much research has been focused on suspended culture systems to examine the performance of anaerobic biohydrogen production using either a single/pure strain and/or a mixed culture.1,2 However, in the case of mixed cultures, several drawbacks have been reported with respect to the low hydraulic retention time (HRT), such as the washout of the biomass and unstable hydrogen-producing bacterial populations.3 Therefore, the stability of the microbial population has become one of the most crucial elements in biohydrogen production.
Some alternative strategies have been proposed, e.g. introducing an attached growth system via an immobilisation technique in an anaerobic fluidised bed (AFBR),4 fixed bed,5 or upflow anaerobic sludge blanket (UASB) reactor6 in order to enhance the biomass retention time. Many studies have demonstrated that the yield and productivity in thermophilic fermentative hydrogen production is higher than in mesophilic hydrogen fermentation.7 However, the lower cell density has been a drawback of fermentation at thermophilic temperatures.8 Therefore, using an attached biofilm instead of suspended growth seems to be very practical in maintaining the culture density and at the same time increasing hydrogen performance.9
Generally, immobilisation involves in the formation of a biofilm or granulation of cells during the fermentation process. This technology has relatively good potential due to the high cell density and adherence to the support material, which avoids cell washout at a low HRT and subsequently improves performance during two-phase separation.9 There exist only a few reports on biohydrogen production using attached growth systems under thermophilic conditions9,10 as most investigators have focused on mesophilic conditions.11,12 A long period of time is required for the granulation process, as reported by Yu and Mu.13 Zhang et al.,14 on the other hand, demonstrated rapid formation of biofilms under anaerobic conditions in a fluidised bed reactor (FBR).
Biofilm formation depends on several factors, such as: (i) the physical surface and chemical composition of the support carrier, (ii) the surrounding environment, such as nutrient availability, pH, and temperature, and (iii) the composition of the microbial consortia.15 Biofilm-based systems have been extensively used as immobilised cell systems as they enhance the reaction rates and population dynamics.16 However, these studies have been limited to the development of mixed microflora biofilms, and the physical conditions have not been well-characterised.17 Further studies on the rapid development of biofilms and the characterisation of such films can minimise the mass transfer resistance and stabilise the hydrogen-producing bacteria on the biofilm for a good hydrogen performance.
In this study, thermophilic fermentative hydrogen production was carried out by immobilization of the anaerobic sludge obtained from a palm oil mill treatment plant on granular activated carbon (GAC) in batch mode. GAC has been known as an inert, hydrophobic sorbent favourable for cell attachment. The effect of pH on biohydrogen production by the thermophilic biofilm was compared with suspended cells (without the support carrier, GAC) using sucrose-containing medium at 60 °C. The characteristics and chemical composition of the GAC-attached biofilm developed under optimum pH conditions was also examined. Finally, the biohydrogen production with this thermophilic biofilm using complex substrate-like palm oil mill effluent (wastewater) as a carbon source was also examined. This knowledge is important for the assessment of reactor performance with real wastewater in future studies.
2. Materials and methods
2.1 Mixed culture, carrier supports, and fermentation medium
The mixed microflora used in this study was obtained from a recent study.18 The anaerobic sludge underwent heat shock (heating at 80 °C for 60 min) and was then acclimatized in a 500 mL anaerobic reactor at 60 °C with a HRT of 48–12 h by feeding palm oil mill effluent in a continuous mode.18 The reactor effluent was collected (5 L) and stored in a laboratory cold room at 4 °C for further use.The carrier used to attach the hydrogen-producing bacteria was granular activated carbon (GAC) grade VISORB with a mesh size of 10 × 16 VS 45 (Carbochem Inc., USA). The GAC was sieved using a sieve shaker (Model EFL 2000/2, Endecotts, London) to obtain the required particle size of 2–3 mm.
The medium used for biohydrogen production contained 10 g L−1 sucrose as the sole carbon and energy source and supplements (in g L−1 unless indicated otherwise) as follows: NH4Cl, 1; NaCl, 2; MgCl2·6H2O, 0.5; CaCl2·2H2O, 0.05; K2HPO4·3H2O, 1.5; KH2PO4, 0.75; NaHCO3, 2.6; cysteine hydrochloride, 0.5; yeast extract, 2; resazurin, 0.5 mg; and trace elements, 1 mL (R&M Chemical, UK).19
2.2 Batch fermentation
In this study, the effect of initial pH on thermophilic fermentative hydrogen production by anaerobic sludge was studied and compared using cells in suspended form as well as attached on granular activated carbon (GAC). The initial pH of the medium was varied from 5.0 to 7.5 with 0.5 increments and was adjusted using either 1 M HCl or 1 M NaOH. Batch cultivation was performed in a 100 mL serum bottle with a working volume of 55 mL and 10% heat-treated POME sludge as the inoculum. Pre-grown, 24 h old culture (5 mL) was added to 50 mL of the aforementioned medium and the serum bottles were purged with nitrogen gas to create anaerobic conditions. The immobilization of the POME sludge was carried out by adding GAC in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of heat treated POME sludge volume (mL) to GAC weight (g) in the serum bottle, while fermentation without GAC was conducted in parallel. The serum bottles were incubated in a shaking water bath (Model SW22, Julabo, Germany) at 150 rpm and 60 °C for 24 hours. The repeated batch cultivation process was continued for a maximum of five successive batches to compare the productivity and stability of hydrogen production at different initial pH values. These experiments were carried out in triplicates.
1 ratio of heat treated POME sludge volume (mL) to GAC weight (g) in the serum bottle, while fermentation without GAC was conducted in parallel. The serum bottles were incubated in a shaking water bath (Model SW22, Julabo, Germany) at 150 rpm and 60 °C for 24 hours. The repeated batch cultivation process was continued for a maximum of five successive batches to compare the productivity and stability of hydrogen production at different initial pH values. These experiments were carried out in triplicates.
The optimal initial pH obtained in the repeated batch cultivation for biohydrogen production was used to conduct another batch study for profiling the cumulative hydrogen productivity and to examine the biofilm formation. The experiment was conducted in a 50 mL serum bottle with a working volume of 25 mL medium. The bacteria grown and attached to the GAC during the final batch of cultivation (initial pH 5.5) were collected and added to 25 mL of fresh medium adjusted to pH 5.5. The ratio of volume of medium (mL) to weight (g) of GAC is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The serum bottles were purged with nitrogen gas to create anaerobic conditions and the serum bottles were incubated in a shaking water bath (Model SW22, Julabo, Germany) at 150 rpm and 60 °C. Samples were analyzed at every 3 hour interval for 48 hours. The biogas produced was sampled using a disposable syringe for further analysis of the gas composition. The supernatant was analysed for soluble volatile fatty acids, total carbohydrates and zeta potential. The biofilm characteristics were determined by compositional analysis of the extracellular polymer substances (EPSs) by chemical extraction and Fourier-transform infrared (FTIR) spectroscopy. Scanning electron microscopy (SEM) was employed for visualization of the biofilm on GAC. The experiment was conducted in triplicate.
1. The serum bottles were purged with nitrogen gas to create anaerobic conditions and the serum bottles were incubated in a shaking water bath (Model SW22, Julabo, Germany) at 150 rpm and 60 °C. Samples were analyzed at every 3 hour interval for 48 hours. The biogas produced was sampled using a disposable syringe for further analysis of the gas composition. The supernatant was analysed for soluble volatile fatty acids, total carbohydrates and zeta potential. The biofilm characteristics were determined by compositional analysis of the extracellular polymer substances (EPSs) by chemical extraction and Fourier-transform infrared (FTIR) spectroscopy. Scanning electron microscopy (SEM) was employed for visualization of the biofilm on GAC. The experiment was conducted in triplicate.
2.3 Calculation of hydrogen production
The cumulative hydrogen production in the batch experiment was determined according to a modified Gompertz equation using Sigma Plot Software 10.0 (Systat Software Inc., USA). Theoretically, the modified Gompertz equation is as follows:20 | (1) |
2.4 Monitoring and analysis of hydrogen gas, VFAs and sugar
Hydrogen gas production was calculated from headspace measurements of the gas composition and the total volume of biogas produced at each time interval was determined using eqn (2):| VH,i = VH,i−1 + CH,i(VG,i − VG,i−1) + VH(CH,i − CH,i−1) | (2) |
The fermentation liquid was filtered through a 0.22 µm syringe filter before analysis. Soluble volatile fatty acids (VFAs) were analysed by HPLC analysis using an Agilent 1100 HPLC system (California, USA) with a REZEX ROA column (Phenomenex, USA), equipped with an ultraviolet (UV) detector. The flow rate of 2.5 mM H2SO4 as the mobile phase was fixed at a constant 0.5 mL min−1 with isocratic elution at 40 °C. Ethanol was determined by HPLC, model Agilent 1200 HPLC system (Califonia, USA) with a REZEX ROA column (Phenomenex, USA) equipped with a refractive index detector (RID), and water as the mobile phase fixed at a constant 0.6 mL min−1 with isocratic elution at 60 °C. The HPLC sample injection volume was 20 µL, and the standard curves were generated using different concentrations of ethanol and mixed organic acids. Sucrose was analysed using the phenol–sulphuric acid method.
2.5 Biofilm extraction, characterization and imaging
The biofilm attached to the carrier (GAC) was mashed with a pestle to detach the extracellular polymeric substance (EPS), placed in a centrifuge tube, and stored at −20 °C for further analysis. The attached EPS was extracted according to the formaldehyde–NaOH method as described by Liu and Fang.22 Prior to extraction, the EPS suspension was prepared by adding 1.5 g (dry weight) of suspended biofilm in 10 mL of ultrapure water (Sartorius, Malaysia). Extraction was performed by treating the sample with 0.06 mL of 37% (w/w) formaldehyde at 4 °C for 1 h. Following the exposure to formaldehyde, the suspension was then treated with 5 mL of 1 M NaOH at 4 °C for about 3 h. Finally, the mixtures were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min to obtain the soluble EPS.
000g for 15 min to obtain the soluble EPS.
Once the EPS was extracted, the soluble EPS was evaluated for carbohydrate content using the phenol–sulphuric acid method. Proteins were determined based on the Bradford method using bovine serum albumin (BSA) as the standard. The total of carbohydrates and proteins was based on the quantity of volatile suspended solids (VSSs) of the biofilm and was measured according to the American Public Health Association (APHA) standard method.
The chemical composition of the extracted EPS was also verified using FTIR spectroscopy (Model Nicolet 6700, Thermo Scientific, USA) via the attenuated total reflectance (ATR) method. The FTIR spectra were acquired in the 4000–400 cm−1 region with a resolution of 2 cm−1 using transmission mode.
The zeta potential was measured using a Zetasizer Nano Particle Analyser (Nano ZS, Model ZEN 3600, Malvern Instrument Ltd., UK) and analysed using Dispersion Technology Software (DTS) version 5.02. The supernatant used for the zeta potential analysis was maintained at about 0.02% on a wet-weight basis with ultrapure water (Sartorius Malaysia Sdn. Bhd., Malaysia).
The biofilm developed on the GAC was visualised using field emission scanning electron microscopy (FESEM) (Model Supra 55VP, Carl Zeiss AG, Germany). The GAC-immobilised cells were fixed with 2% (w/w) glutaraldehyde and left overnight at 4 °C. The fixed samples were washed with 0.1 M phosphate buffer solution three times and left for 10 min each. Dehydration was carried out by successive passages through 30, 50, 70, 80, 90, and 100% (w/w) alcohol. The dehydrated particles were then transferred to a Critical Point Dryer (Model Leica EM CPD 300, Leica Microsystems, Germany) for 1 h and 30 min. The dried samples were sputter-coated with platinum and finally analysed using FESEM.
2.6 Validation of the GAC-attached thermophilic biofilm using POME as a substrate
In order to validate the efficiency of the GAC-attached thermophilic biofilm to produce hydrogen from palm oil mill effluent (POME), batch experiments were carried out in 50 mL serum bottles of 25 mL of working volume. For this purpose, the synthetic medium mentioned in Section 2.1 was amended by adding raw POME (10% v/v) and replacing 10 g L−1 sucrose with glucose and xylose (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), mimicking sugars available in raw POME. The hydrogen production performance was also compared to that using synthetic medium without the addition of POME but with mixed sugars (Glucose and xylose) in the same ratio. The pH of the medium was adjusted to 5.5 and it was purged with nitrogen gas to create anaerobic conditions. The serum bottles were incubated in a shaking water bath (Model SW22, Julabo, Germany) at 150 rpm and 60 °C. Analyses of biogas, soluble volatile fatty acids, and total carbohydrates were carried out as described in Section 2.4.
1), mimicking sugars available in raw POME. The hydrogen production performance was also compared to that using synthetic medium without the addition of POME but with mixed sugars (Glucose and xylose) in the same ratio. The pH of the medium was adjusted to 5.5 and it was purged with nitrogen gas to create anaerobic conditions. The serum bottles were incubated in a shaking water bath (Model SW22, Julabo, Germany) at 150 rpm and 60 °C. Analyses of biogas, soluble volatile fatty acids, and total carbohydrates were carried out as described in Section 2.4.
3. Results and discussion
3.1 Batch cultivation
The effect of the initial pH of the mixed culture from heat treated POME sludge with GAC and without GAC (control) was studied in the pH range of 5.0–7.5. The effect of initial pH in hydrogen fermentation is a crucial aspect because inappropriate pH could inhibit hydrogenase activity.23 The results shown in Fig. 1 are the steady-state data of the hydrogen performance of the last batch at different initial pH values. | ||
| Fig. 1 Hydrogen production rate and hydrogen content of final batch cultivation with GAC and without GAC (control) at various initial pH values. | ||
With the suspended cells, the biohydrogen production rate gradually increases from 2.82 to 4.28 mmol H2 L−1 h−1 when the initial pH increases from 5.0 to 6.5. However, a further increase in the initial pH to 7.0 reduced the biohydrogen production rate two-fold compared to that in pH 5.0. A maximum H2 content of 47.8% was obtained at pH 5.5. Generally, pH values between 5.0 and 6.5 are reported to be favorable for better microbial activity and hence better hydrogen production.24
With the GAC-attached biofilm, both the H2 production rate and H2 content increased when increasing the initial pH from 5.0 to 5.5. With a further pH increase, the performance declined. The highest H2 production rate and H2 content (5.32 mmol H2 L−1 h−1 and 51.2%, respectively) were obtained at an initial pH of 5.5 with GAC. In this study, the biohydrogen production rate could be enhanced up to 51% by using GAC as a support material at an initial pH of 5.5. The optimal cultivation pH of 5.5 for GAC is in agreement with reports from other researchers on thermophilic hydrogen fermentation at 60 °C.8
Furthermore, the maximum hydrogen production rate at initial pH values of 7.0 and 7.5 for the control experiment shows the lowest value among the initial pH values, with approximately 1.0 mmol H2 L−1 h−1. This observation is similar to that of Chong et al. (2013), who also reported that the growth of bacteria as well as hydrogen production is restricted at pH values above 7.0 in suspended culture.25 However, it is interesting to note that a higher value for hydrogen production was recorded at an initial pH of 7.0 (4.3 mmol H2 L−1 h−1) in the presence of GAC in this study than those recorded at initial pH 5.0–6.5 (which were in the range of 2.82–4.28 mmol H2 L−1 h−1) in the control experiment (Fig. 1). This is due to the mechanical stability of the biofilms formed on the GAC, which have a great binding capacity for organic matter, and hence provide an environment that is rich in nutrients, promoting microbial adhesion.26
Therefore the experiment in the presence of GAC even under non-favorable pH conditions exhibited better hydrogen production than the suspended cells in the control experiments in all different initial pH values studied. The immobilization system enabled the cells to withstand considerable shear force and stay active towards a stressful environment.26
From the microbiological point of view, the suitable pH at which hydrogen production would be optimum is subject to the type of hydrogen producing bacteria (HPB) in the inoculum. In our recent study18 focusing on the community analysis of the anaerobic sludge from a palm oil mill treatment plant, it was shown that the reactor effluent harbours species of Bacillus as the dominant culture. Bacillus smithii CMB-B1 and Bacillus coagulans M36 species have been identified to be responsible for the production of hydrogen.
The same sludge was employed in this present study. Generally, bacilli thermophilic (thermophilic bacilli) are aerobic or facultative anaerobic bacteria that live in the temperature range of 45–70 °C. Bacillus species have already been reported as producing hydrogen, in addition to Enterobacter and Clostridium species.27
The temperature range and optimum pH for growth and hydrogen production of Bacillus smithii have been reported to be 25 °C to 60 °C and pH 5.7 by Nakamura et al. (1988).28 The potential of Bacillus smithii as hydrogen-producing bacteria was also studied by Grady et al. (1998), who used this species to convert waste biomass into hydrogen.29 The Bacillus coagulans species has also been identified as a hydrogen producer under either mesophilic and thermophilic conditions.30
3.2 Kinetic analysis of batch fermentation using GAC-immobilized cells
Fig. 2 shows the kinetic analysis of biohydrogen and soluble metabolite production at initial pH 5.5 using GAC-attached POME sludge. The comparison between the experimental data and the predicted modified Gompertz model of the cumulative hydrogen production of the GAC-attached biofilm is shown in Fig. 2(a). The kinetic parameters obtained were based on the cumulative hydrogen production data fitted by the modified Gompertz model with Hm = 58.6 mL, Rm = 2.8 mL h−1, and λ = 0.003 h. From the results, the hydrogen production rate (HPR) obtained was 4.3 mmol L−1 h−1. An experimental H2 yield of 5.6 mol H2 per mol sucrose, which equals 2.8 mol H2 per mol hexose, was obtained by using the acclimatized GAC-attached biofilm as inocula.The concentration of soluble metabolite products (SMPs) (as shown in Fig. 2(b)) increased with an increase in the fermentation time, which was reflected in greater hydrogen production, as shown in Fig. 2(a). The primary SMP was acetic acid (HAc), comprising 40–55% of the SMPs, followed by butyric acid (HBu), which accounted for approximately 30–40% of the fermentation liquor. In contrast, the production of ethanol (EtOH), which is considered an unfavourable metabolite for hydrogen production, accounted for less than 20% of the SMPs. The amount of acetic acid produced at 48 h was 24.2 mM, followed by 22.5 mM butyric acid and 11 mM ethanol. The prevalence of acetic acid and butyric acid in the culture supernatant suggested that the acidogenesis pathway was favoured to produce greater amounts of biohydrogen.31 The study by Kotay and Das (2007) using Bacillus coagulans IIT-BT SI isolated from sewage sludge also produced acetic acid, butyric acid and ethanol as the primary metabolites during fermentative biohydrogen production.30
In this study, a higher yield of H2 is obtained by using GAC-attached POME sludge in a batch cultivation process under thermophilic conditions, compared to Wu et al.31 who used an acclimatized, attached biofilm under mesophilic conditions. The H2 evolution was rapid with no lag time and the hydrogen content was 43.8% of the total biogas produced, with an almost threefold working volume of fermentation. No methane was detected throughout the experiment.
Table 1 summarizes similar studies on biohydrogen production from mixed cultures attached on activated carbon using sucrose as the sole carbon source. Even though the carrier material and carbon source used are similar for all these studies, the current study seems to be unique in developing a bioprocess for hydrogen production by an acidogenic, thermophilic biofilm on GAC. It also revealed that by employing a repeated batch system, the hydrogen yield, even on a smaller scale (serum bottle), can be improved up to 3–4 fold compared to Wu et al.31 Further improvements on the fermentation performance can be achieved by using bioreactor systems where the operational parameters, such as pH and temperature, can be controlled throughout the experiment.
| Microorganisms | Substrate | Immobilization carrier | Mode/process | Initial pH | Optimal operation temperature (°C) | Max. H2 yield (mol H2 per mol hexose) | Reference |
|---|---|---|---|---|---|---|---|
| a CIGSB – carrier-induced granular sludge bed; CSABR – continuously stirred anaerobic bioreactor; FBR – fluidized-bed reactor. | |||||||
| Sewage sludge/mixed culture | Sucrose | Activated carbon | Continuous/fixed-bed | 6.7 | 35 | 0.59 | 5 |
| Sewage sludge/mixed culture | Sucrose | Activated carbon | Continuous/packed-bed | 6.7 | 35 | 1.45 | 32 |
| Sewage sludge/mixed culture | Sucrose | Activated carbon | Continuous/CIGSB | 6.7 | 35 | 1.5 | 33 |
| Sewage sludge/mixed culture | Sucrose | Activated carbon | Continuous/CSABR | 6.6 ± 0.2 | 40 | 1.93 | 7 |
| Sewage sludge/mixed culture | Sucrose | Activated carbon | Batch/serum vial | 6.7 | 40 | 0.87 | 31 |
| Sewage sludge/mixed culture | Sucrose | Activated carbon | Continuous/FBR | 6.4 | 40 | 1.88 | 11 |
| POME sludge/mixed culture | Sucrose | Granular activated carbon | Batch/serum bottle | 5.5 | 60 | 2.80 | This study |
The total concentration of volatile fatty acids (TVFAs) achieved at initial pH 5.5 after 48 h fermentation in the presence of GAC was 46.7 mmol TVFAs (referring to the total amount of acetic and butyric acid) and hence contributed to a higher hydrogen performance. Immobilization of the hydrogen-producing bacteria on GAC provided protection from exposure to metabolite accumulation, acidic conditions, and low substrate concentration during fermentation.
3.3 Biofilm characterization and imaging
Biofilms are complex assemblages of microorganisms that are embedded in a matrix of extracellular polymeric substances (EPSs). During the repeated batch cultivation, mixed microbial cells grew in association with the activated carbon surfaces and resulted in the formation of a biofilm. The characterization of the biofilm was carried out by extracting the EPS using chemical extraction, followed by chemical content analysis, zeta potential measurements and observation using FESEM.In this study, extraction was carried out using the formaldehyde–NaOH method of Liu and Fang as it is the most effective extraction method.22 The compositions of total carbohydrate (TC) and protein in the extracellular polymeric substances (EPSs) of the GAC-immobilised-cell biofilm at different time intervals of fermentation are shown in Table 2. As can be seen in Table 2, the carbohydrate and protein levels in the extracted EPS constituents increased with an increase in the fermentation time, which may have been the result of enhanced adhesion between the bacteria and GAC during biofilm formation. At the beginning of the fermentation (3 hour), both carbohydrate and protein contents were low. However, after 48 h of fermentation, carbohydrate and protein comprised 9.2 mg g−1 VSS (40%) and 13.2 mg g−1 VSS (60%) of the extracted EPS, respectively. The ratio of protein to carbohydrate (P/C) in the EPS for all contact times varied between 1.4 and 2.6. Similar results for protein/polysaccharide ratios of biofilm EPS between 1.8 and 5.4 were reported by Ras et al.34
| Time (h) | Total EPS composition (mg g−1 VSS) | Ratio | |
|---|---|---|---|
| Carbohydrate | Protein | Protein/carbohydrate | |
| 3 | 3.8 ± 0.4 | 9.8 ± 0.8 | 2.6 |
| 12 | 7.3 ± 0.3 | 10.6 ± 0.9 | 1.5 |
| 24 | 7.7 ± 0.2 | 12.3 ± 0.5 | 1.6 |
| 48 | 9.2 ± 0.3 | 13.2 ± 0.4 | 1.4 |
Moreover, there seemed to be a direct relationship between the increase in total EPS concentration (given in Table 2) and the total soluble metabolites produced (as shown in Fig. 2(b)) during hydrogen production. In this study, the total EPS concentration increased from 13.6 mg g−1 VSS after 3 h fermentation to 22.4 mg g−1 VSS after 48 h fermentation, which can be correlated with the increase in total SMP (SMP = TVFA + EtOH) from 9.1 mM to 57.7 mM over 48 h of fermentation. The EPS secreted by mixed microflora can be subdivided into bound EPS (e.g., attached organic materials) and soluble EPS, sometimes referred to as soluble microbial products (SMPs).35 It is well established that the production of this SMP reflects the hydrogenase metabolic pathway and hence, the performance of hydrogen production.23 Thus, the EPS concentration plays an important role in establishing the structural and functional integrity of microbial biofilms and exhibits a direct relationship with hydrogen production.36 However, the types of SMPs and the degree of microbial adhesion on the immobilized carrier will vary depending on the microbial species and operational conditions, such as temperature and pH.
Fig. 4 shows changes in the zeta potential and pH of the culture supernatant during biohydrogen production under thermophilic conditions. Before immobilization and cultivation, the zeta potential of the raw sludge from POME at pH 5.5 was −2.04 mV.
 | ||
| Fig. 4 Zeta potential and pH of supernatant at different time intervals during biohydrogen production. | ||
After cultivation, the zeta potential or surface charge of the supernatant decreased to a negative value with increasing fermentation time. Just after 3 h of fermentation, the zeta potential was slightly reduced from −2.04 mV to −2.58 mV, and finally to −9.17 mV after 48 h, at the end of batch fermentation. The culture pH was shown to display a similar trend to the zeta potential as the pH value decreased from an initial pH of 5.5 to 4.74.
In contrast, Lin et al.,40 reported that the zeta potential of the pure culture increased with decreasing culture pH. A similar phenomenon was observed by Zhang et al.41 during biofilm formation under acid incubation. In their study, an increase in the zeta potential of GAC from 2.74 mV to 76.41 mV accompanied a reduction in pH from 5.5 to 2.0. However, it is worthy to note that, in the present study, the pH fluctuation was not as abrupt as in other studies and hence both pH and zeta potential showed similar trends. In addition, the increase in EPS content with increasing fermentation time (as shown in Table 2) suggested that the culture zeta potential could be influenced by adsorbing EPS that carried more negative charges.14
Recently, Su et al.39 have demonstrated that the decrease in zeta potential may speed up the granulation progress as a necessary condition. Bacterial adhesion is determined by an interplay between hydrophobic and electrostatic interactions. When bacteria approach the surface of the support material, they experience an electrostatic repulsion since both the bacteria and the GAC particle surface are negatively charged.14 However, as the zeta potential decreases, microbial aggregation tends to strengthen because the low zeta potential reduced the repulsive electrostatic interactions. According to Gottenbos et al.,42 a positively charged surface adversely affects biofilm formation. Thus, a negative surface charge as in cultivated sludge POME is an advantage for biofilm formation.
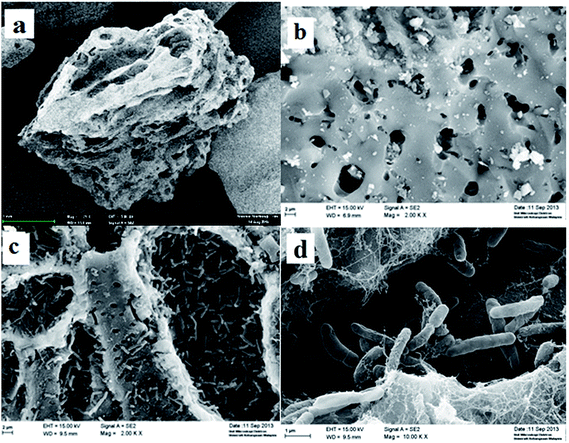
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification) in Fig. 5(d) revealed the attachment of individual cells onto the surfaces as well as within the cavities of GAC. These SEM images indicated that a stable and successful immobilization was achieved under thermophilic conditions. The surface porosity of GAC facilitated the attachment of bacteria to the surface with the aid of the conditioning layer and the EPS formation at the substratum. EPS at the substratum minimised the mass transfer resistance and stabilised the hydrogen-producing bacteria on the biofilm to give a good hydrogen performance.
000× magnification) in Fig. 5(d) revealed the attachment of individual cells onto the surfaces as well as within the cavities of GAC. These SEM images indicated that a stable and successful immobilization was achieved under thermophilic conditions. The surface porosity of GAC facilitated the attachment of bacteria to the surface with the aid of the conditioning layer and the EPS formation at the substratum. EPS at the substratum minimised the mass transfer resistance and stabilised the hydrogen-producing bacteria on the biofilm to give a good hydrogen performance.
3.4 Validation of GAC-attached thermophilic biofilm using POME as a substrate
Sucrose (10 g L−1) has been used throughout the study to establish whether or not the GAC-immobilized system developed herein is successful for producing biohydrogen under thermophilic conditions. We have also examined biohydrogen production with this thermophilic biofilm using diluted palm oil mill effluent (POME) as the carbon source. The POME used in this study had total suspended solids of about 47.9 ± 0.4 g L−1. Generally, the attached growth system is not suitable for substrates with high solid contents. Therefore, we attempted to use the diluted POME to study the performance of the GAC-attached thermophilic biofilm for biohydrogen production. Fig. 6 shows the experimental data and predicted profile from the modified Gompertz model of cumulative hydrogen production from POME with GAC-attached cells at 60 °C. The maximum hydrogen produced, Hm, in 25 mL of medium is 40.3 mL or is equivalent to an Rm of 4.8 mL h−1. This is slightly lower than the control (Hm of 44.3 mL and Rm of 5.7 mL h−1) which consisted of 100% synthetic medium and glucose and xylose mimicking the sugar composition of raw POME. | ||
| Fig. 6 Batch kinetics of hydrogen production from palm oil mill effluent (POME) with GAC-attached biofilm at 60 °C. | ||
In this study, the hydrogen yield in the presence of POME was 1.75 mol H2 per mol sugar consumed, whereas the maximum hydrogen production rate was 4.1 mmol H2 L−1 h−1. The concentration of soluble metabolite products (SMPs) produced was 33.3 mM, with acetate and butyrate comprising 99% of the total SMP, with 1% ethanol. The dominance of acetate and butyrate formation indicated that the pathway of hydrogen production is the acidogenic pathway. A lower hydrogen yield was obtained when using POME (1.75 mol H2 per mol sugar) compared to sucrose (2.8 mol H2 per mol hexose) (Section 3.2), suggesting that the GAC-immobilized cells need some adaptation time to a new combination of carbon sources, which consisted of hexose (glucose) and pentose (xylose).43 Nevertheless, the hydrogen production rate of POME (4.1 mmol L−1 h−1) was almost similar to that when using sucrose (Section 3.2), which was 4.3 mmol L−1 h−1, probably because the culture used in this study has already been acclimatized in POME. Moreover, as fermentation was performed under optimum pH and temperature, the enriched community remained the same over the cultivation period with consistent fermentation performance.
4. Conclusions
The biohydrogen production performance of suspended sludge and immobilized cell systems with GAC were investigated in this study. The optimal initial pH for thermophilic biohydrogen production was found to be 5.5. Adding GAC resulted in prompt microbial colonization and biofilm development with a H2 yield of 5.6 mol H2 per mol sucrose and a hydrogen production rate of 4.3 mmol L−1 h−1. This may be due to the protective effect of the GAC carrier of the attached growth biofilm against the acidic environment, in contrast with the suspended culture. Further improvements on the thermophilic biohydrogen production by GAC-immobilized cells are currently being attempted in a fluidised bed anaerobic bioreactor to examine the microbial adhesion and tolerance at low hydraulic retention time (HRT).Acknowledgements
The authors would like to acknowledge the Universiti Kebangsaan Malaysia – Yayasan Sime Darby (UKM-YSD) Chair for Sustainable Development for Palm Oil Industry endowment fund and research grant under code DIP-2014-030 Universiti Kebangsaan Malaysia for financial support. The authors also appreciate positive comments and suggestions given by the Chair Holder of UKM-YSD (2011–2014), Dr Pieternel Claassen, for better presentation of our manuscript.References
- I. Ismail, M. A. Hassan, N. A. A. Rahman and C. S. Soon, Biomass Bioenergy, 2010, 34, 42–47 CrossRef CAS PubMed.
- P. M. Abdul, J. M. Jahim, S. Harun, M. Markom, O. Hassan, A. W. Mohammad and A. J. Asis, Int. J. Hydrogen Energy, 2013, 38, 15693–15699 CrossRef CAS PubMed.
- M. Badiei, J. M. Jahim, N. Anuar and S. R. S. Abdullah, Int. J. Hydrogen Energy, 2011, 36, 5912–5919 CrossRef CAS PubMed.
- Z. P. Zhang, K. Y. Show, J. H. Tay, D. T. Liang and D. J. Lee, Int. J. Hydrogen Energy, 2008, 33, 1559–1564 CrossRef CAS PubMed.
- J. S. Chang, K. S. Lee and P. J. Lin, Int. J. Hydrogen Energy, 2002, 27, 1167–1174 CrossRef CAS.
- H. Q. Yu and Y. Mu, Biotechnol. Bioeng., 2006, 94, 988–995 CrossRef CAS PubMed.
- F. Kargi, N. S. Eren and S. Ozmihci, Int. J. Hydrogen Energy, 2012, 37, 8338–8342 CrossRef CAS PubMed.
- S. Othong, P. Prasertsan, D. Karakashev and I. Angelidaki, Int. J. Hydrogen Energy, 2008, 33, 6498–6508 CrossRef CAS PubMed.
- T. Keskin, E. Aksöyek and N. Azbar, Int. J. Hydrogen Energy, 2011, 36, 15160–15167 CrossRef CAS PubMed.
- M. A. Basile, C. Carfagna, P. Cerruti, G. G. d’Ayala, A. Fontana, A. Gambacorta, M. Malinconico and L. Dipasquale, RSC Adv., 2012, 2, 3611–3614 RSC.
- S. Y. Wu, C. Y. Chu and Y. C. Shen, Int. J. Hydrogen Energy, 2012, 37, 15496–15502 CrossRef CAS PubMed.
- S. Y. Wu, C. H. Hung, C. N. Lin, H. W. Chen, A. S. Lee and J. S. Chang, Biotechnol. Bioeng., 2006, 93, 934–946 CrossRef CAS PubMed.
- H. Q. Yu and Y. Mu, Biotechnol. Bioeng., 2006, 94, 988–995 CrossRef CAS PubMed.
- Z. P. Zhang, K. Y. Show, J. H. Tay, D. T. Liang, D. J. Lee and W. J. Jiang, Biotechnol. Bioeng., 2007, 96, 1040–1050 CrossRef CAS PubMed.
- E. D. L. Pulcini, Nephrologie, 2001, 22, 439–441 Search PubMed.
- V. Lazarova and J. Manem, Innovative biofilm treatment technologies for water and wastewater treatment, in Biofilms II: process analysis and applications, Wiley, New York, 2000, pp. 159–206 Search PubMed.
- S. Wuertz, S. Okabe and M. Hausner, Water Sci. Technol., 2004, 49, 327–336 CAS.
- N. Ibrahim, MSc thesis, Universiti Kebangsaan Malaysia, 2013.
- I. Angelidaki and W. Sanders, Environ. Sci. Biotechnol., 2004, 3, 117–129 CrossRef CAS PubMed.
- W. H. Chen, S. Y. Chen, S. Khanal and S. Sung, Int. J. Hydrogen Energy, 2006, 31, 2170–2178 CrossRef CAS PubMed.
- S. W. Van Ginkel, S. E. Oh and B. Logan, Int. J. Hydrogen Energy, 2005, 30, 1535–1542 CrossRef CAS PubMed.
- H. Liu and H. H. P. Fang, J. Biotechnol., 2002, 95, 249–256 CrossRef CAS.
- D. Infantes, A. G. Campo, J. Villaseñor and F. J. Fernández, Int. J. Hydrogen Energy, 2011, 36, 15595–15601 CrossRef CAS PubMed.
- P. Sinha and A. Pandey, Int. J. Hydrogen Energy, 2011, 36, 7460–7478 CrossRef CAS PubMed.
- P. S. Chong, J. M. Jahim, S. Harun, S. S. Lim, S. A. Mutalib, O. Hassan and M. T. M. Nor, Int. J. Hydrogen Energy, 2005, 30, 1535–1542 CrossRef PubMed.
- G. P. Sheng, H. Q. Yu and X. Y. Li, Biotechnol. Adv., 2010, 28, 882–894 CrossRef CAS PubMed.
- R. Nandi and S. Sengupta, Crit. Rev. Microbiol., 1998, 24, 61–84 CrossRef CAS PubMed.
- L. K. Nakamura, I. Blumenstock and D. Claus, Int. J. Syst. Evol. Microbiol., 1988, 38, 63–73 CAS.
- J. L. Grady and G. J. Chen, US Pat., US5821111A, 1998.
- S. M. Kotay and D. Das, Bioresour. Technol., 2007, 98, 1183–1190 CrossRef CAS PubMed.
- S. Wu, C. Lin and J. Chang, Int. J. Hydrogen Energy, 2005, 30, 1375–1381 CrossRef CAS PubMed.
- K. S. Lee, Y. S. Lo, Y. C. Lo, P. J. Lin and J. S. Chang, Biotechnol. Lett., 2003, 25, 133–138 CrossRef CAS.
- K. S. Lee, J. F. Wu, Y. S. Lo, Y. C. Lo, P. J. Lin and J. S. Chang, Biotechnol. Bioeng., 2004, 87, 648–657 CrossRef CAS PubMed.
- M. Ras, D. Lefebvre, N. Derlon, E. Paul and E. G. Neuhauser, Water Res., 2011, 45, 1529–1538 CrossRef CAS PubMed.
- S. Rosenberger and M. Kraume, Desalination, 2002, 146, 373–379 CrossRef CAS.
- S. S. Branda, A. Vik, L. Frieldman and R. Kolter, Trends Microbiol., 2005, 13, 20–26 CrossRef CAS PubMed.
- A. Iyer, K. Mody and B. Jha, Indian J. Exp. Biol., 2005, 43, 467–471 CAS.
- M. A. Kumar, K. T. K. Anandapandian and K. Parthiban, Braz. Arch. Biol. Technol., 2011, 54, 259–265 CrossRef CAS PubMed.
- B. Su, Z. Qu, Y. Song, L. Jia and J. Zhu, J. Environ. Chem. Eng., 2014, 2, 1142–1147 CrossRef CAS PubMed.
- D. Q. Lin, L. N. Zhong and S. J. Yao, Biotechnol. Bioeng., 2006, 95, 185–191 CrossRef CAS PubMed.
- Z.-P. Zhang, K.-Y. Show, J.-H. Tay, D. T. Liang, D.-J. Leed and A. Sue, Int. J. Hydrogen Energy, 2008, 33, 5151–5160 CrossRef CAS PubMed.
- B. Gottenbos, D. W. Grijpma, V. D. H. C. Mei, J. Feijnen and H. J. Busscher, J. Antimicrob. Chemother., 2001, 48, 7–13 CrossRef CAS.
- A. A. Abreu, J. I. Alves, M. A. Pereira, D. Karakashev, M. M. Alves and I. Angelidaki, Bioresour. Technol., 2010, 101, 9577–9586 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N + N–H groups in proteins, and (c) unsaturated bonds.
O and C–N + N–H groups in proteins, and (c) unsaturated bonds.