Green synthesis of gold nanoparticles and its application for the trace level determination of painter's colic†
Chelladurai Karuppiaha,
Selvakumar Palanisamya,
Shen-Ming Chen*a,
R. Emmanuelb,
K. Muthupandib and
P. Prakash*b
aElectroanalysis and Bioelectrochemistry Lab, Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, No. 1, Section 3, Chung-Hsiao East Road, Taipei 106, Taiwan, Republic of China. E-mail: smchen78@ms15.hinet.net; Fax: +86-2-27025238; Tel: +86-2-27017147
bDepartment of Chemistry, Thiagarajar College, Madurai-625009, Tamilnadu, India. E-mail: kmpprakash@gmail.com; Fax: +91-4522312375; Tel: +91-9842993931/+91-4522456783
First published on 19th January 2015
Abstract
The green synthesis of metal nanoparticles is found to be more attractive in various disciplines, including analytical chemistry. The present study demonstrates a selective voltammetric determination of painter's colic (lead poisoning) using green synthesized gold nanoparticles (Au-NPs) modified glassy carbon electrode (GCE). The Au-NPs were synthesized by the reduction of chloroaurate ions with the fresh leaf extract of Justicia glauca. The formation of Au-NPs was confirmed by UV-visible spectroscopy using surface plasmon resonance. The average size of the synthesized Au-NPs is found to be 32.5 ± 0.25. A good cathodic response current was observed at the Au-NPs modified electrode, whereas the unmodified electrode does not show any response in the presence of Pb2+. With optimum condition, the Au-NPs modified electrode exhibits a good response towards Pb2+ with a linear response range from 0.005 to 800 μM L−1 and the lowest detection limit of 0.07 nM L−1. The fabricated sensor exhibits a high selectivity towards Pb2+ in the presence of 100 fold concentrations of other metal ions. In addition, the proposed sensor also shows a good practicality towards Pb2+ in river water samples.
1. Introduction
The lead ion (Pb2+) is considered one of the most common toxic heavy metals, which accumulates easily in plants and animals.1 Pb2+ can also have adverse effects on human health and the environment, resulting in neurological, cardiovascular and developmental disorders, even at very low levels. Lead poisoning (also known as plumbism, colica pictorum, saturnism, Devon colic, or painter's colic) is a type of metal poisoning in humans and other vertebrates caused by increased levels of lead in the body. Lead interferes with a variety of body processes and is toxic to many organs and tissues, including the heart, bones, intestines, kidneys, and reproductive and nervous systems. Lead interferes with the development of the nervous system and is particularly toxic to children, causing potentially permanent learning and behavior disorders.2 A recent report by the CDC (Centers for Disease Control and Prevention-2014) Childhood Lead Poisoning Prevention Program suggests that even a blood level of 10 micrograms per decilitre can have harmful effects on children's learning behavior. No safe threshold for lead exposure has been discovered, i.e., there is no known sufficiently small amount of lead that will not cause harm to the body.3 According to the US Environmental Protection Agency (EPA), the maximum contamination level for Pb2+ in potable water is approximately 75 nM L−1. Hence, the monitoring of Pb2+ concentrations below this level is a highly challenging task.3 Over the past few decades, many methods have been developed for the trace level detection of Pb2+.4 The electrochemical detection of Pb2+ has attracted considerable attention due to its simplicity and portability compared to traditional methods, such as inductively coupled plasma mass spectrometry (ICPMS), thermal ionization mass spectrometry (TIMS) and X-ray fluorescence spectrometry (XRF).5 Recently, many sensitive and selective Pb2+ sensors have been developed using different modifiers on electrodes, such as enzymes, carbon nanomaterials, polymers, and functional nanoparticles.6–11 Metal nanoparticles have attracted particular interest in the detection of Pb2+ owing to their unique electronic, magnetic, catalytic and optical properties, which are different from bulk metals and dependent on their size and shape.12In particular, gold nanoparticles (Au-NPs) are one of the emerging and predominant metal nanoparticles, which play important roles in many research areas including heavy metal ion determination.13,14 An extensive number of studies and manufacturing procedures have been put into practice for the synthesis of Au-NPs, comprising a variety of physical and chemical methods, electrochemical reduction, heat evaporation, photochemical reduction, laser ablation, inert gas condensation, thermolysis, and radiolysis.15,16 However, the currently available physical methods have a low production rate of Au-NPs with high expenditure.15 In addition, chemical methods involve toxic chemicals that will be adsorbed on the surface of Au-NPs. Furthermore, the large scale production of Au-NPs is impractical because of certain drawbacks, such as polydispersity and stability. Therefore, there is an increasing need to develop high-yield, low cost, non-toxic and environmentally benign procedures for the synthesis of Au-NPs. Currently, the implementation of an alternative options, i.e. a greener approach, has become a promising method for the synthesis of Au-NPs. In a previous study, Au-NPs were synthesized using an Acacia nilotica twig bark extract as a biomaterial.17 In the present study, Au-NPs were synthesized extracellularly using a Justicia glauca leaf extract. The species Justicia is one of the largest species of Acanthaceae, with approximately 600 species found as perennial herbs or sub shrubs in pan tropical and tropical regions. Justicia glauca mainly contains water-soluble heterocyclic compounds, such as lignans, alkaloids, flavonoids, steroids and terpenoids.18,19 These biomolecules in leaf extracts are responsible for the reduction of chloroaurate ions. Recently, Au-NPs have been used as a modifier for the sensing of heavy metals, including green derived metal nanoparticles.13,14,20 In an earlier report, we demonstrated the green synthesis of Au-NPs using a plant extract for the electrochemical determination of nitrobenzene.17 However, there are no reports on the selective electrochemical determination of Pb2+ using Au-NPs. To the best of our knowledge, this is the first report of the selective trace level detection of Pb2+ using green derived Au-NPs.
This paper reports the green synthesis of Au-NPs using Justicia glauca leaf extract as a reducing agent. The green synthesized Au-NPs modified glassy carbon electrode (GCE) was used further for the selective determination of Pb2+. The essential parameters towards the response to Pb2+, such as solution pH, accumulation potential and the accumulation time were optimized and are discussed in detail. The proposed sensor showed good analytical performance (limit of detection and linear response range) towards Pb2+ that was comparable to recently reported Pb2+ sensors (Table 2). In addition, the selectivity and practicality of the Au-NPs modified electrode toward the determination of Pb2+ is also discussed.
| Band positions (cm−1) | Assignment31–37 | |
|---|---|---|
| (a) | (b) | |
| 3752 and 3652 | 3752 and 3652 | –C– of carboxylic group |
| 3371 | 3435 | Hydroxyl groups |
| 2925 and 2850 | 2925 and 2850 | –C–H– group in aromatic ring |
| 1741 | 1767 | Carbonyl group as in aldehydic or ketonic group |
| 1649 | 1767 | Hydroxyl groups in aromatic ring |
| 1514 | 1599 | C–C aromatic group |
| 1400 | 1425 | C–H group in aromatic ring |
| 1153 | 1153 | Carbonyl group as in aldehydic or ketonic group |
| 1028 | 1018 | C–O–C of phenolic |
2. Experimental
Materials
Chloroauric acid (HAuCl4·3H2O) was purchased from Sigma-Aldrich and used as received. The leaves of Justicia glauca were collected from the Sirumalai Hills region, Tamil Nadu, India. The supporting electrolyte pH 5 (acetate buffer) solution was prepared using 0.05 M CH3COOH and CH3COONa solutions in doubly distilled water. All the chemicals used in this study were of analytical grade and all the solutions were prepared using doubly distilled water without further purification.Methods
The size and morphology of the Au-NPs were examined by transmission electron microscopy (TEM, JEOL JEM 2100) model on a JEOL JEM 2100 instrument. Energy Dispersive X-ray (EDX) spectrometer attached to the transmission electron microscope was used for elemental analysis. Fourier transform infrared spectroscopy (FTIR) was carried out using the Shimadzu FTIR-8201 PC instrument. A Jasco V-560 double-beam spectrophotometer was used for UV-visible spectral analysis. X-ray diffraction (XRD, PW3050/60) was performed on the purified Au-NPs with data analysis by XPERT-PRO. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were employed using a computerized electrochemical workstation CHI 750a workstation. A conventional three-electrode assembly, consisting of a modified glassy carbon electrode as the working electrode, an Ag/AgCl electrode (Sat. KCl) as the reference electrode and 0.5 mm diameter platinum wire as the counter electrode, was used for the electrochemical experiments.Preparation of the Justicia glauca leaf extract
The leaves of Justicia glauca were cleaned to remove the adhering mud particles as well as possible impurities. Subsequently, the leaves were laid on the filter paper to eliminate any moisture content in the leaves and air-dried at room temperature for one hour. 0.300 g leaves were weighed and cut into tiny pieces. Afterward, the leaves were boiled with 300 mL of sterile distilled water in an Erlenmeyer flask for 15 min and allowed to cool to room temperature. The boiled and cooled leaf extract was doubly filtered. The leaf extract was pale yellowish green in color and was shining with a fluorescent color similar to sunlight.Green synthesis of Au-NPs
For the synthesis of Au-NPs, 100 mL of an aqueous solution of 1 mM chloroauric acid was added to the Erlenmeyer flask containing 100 mL of Justicia glauca leaf extract. After 10 min, the leaf extract turned a pink red indicting the formation of Au-NPs. The pink color of the aqueous gold colloids became deeper, and after an hour, there was no noticeable difference in the color of the aqueous gold colloids. The color changes indicate the formation of Au-NPs in an aqueous solution due to excitation of the surface plasmon vibration in the metal nanoparticles. Later, the Au-NPs were centrifuged and washed three times with deionized water. After lyophilisation, the Au-NPs were stored in a screw cap bottle for further characterization.Fabrication of Au-NPs modified GCE
Prior to the fabrication of the Au-NPs modified GCE, Au-NPs dispersion was prepared by sonicating Au-NPs (3 mg mL−1) for 45 min in doubly distilled water. Subsequently, 10 μL (optimum) of Au-NPs dispersion was drop cast onto GCE and allowed to dry at room temperature. Finally, the fabricated Au-NPs modified GCE was used for all the electrochemical experiments and was stored at room temperature when not in use. All the electrochemical studies were performed in a pH 5 solution under a N2 atmosphere to avoid the diffusion of atmospheric oxygen into the electrolyte solution.3. Results and discussion
Characterization of synthesized Au-NPs
UV-vis spectroscopy is an excellent techniques for determining the formation of metal nanoparticles provided surface plasmon resonance (SPR) exists for the metal. It was recorded from aliquots that were isolated from aqueous gold colloid mixtures at different time intervals of the bioreduction reaction. The formation of the Au-NPs was principally identified by the change in color from the pale yellowish green leaf extract to a pink-red color within 10 min. The time taken for a complete reduction was 1 h. The absorption reached a constant value after 1 h. When the stability was checked after one month, there was no change in absorption, which indicates that the nanoparticles are stable, even up to one month under ambient conditions (28 °C).Fig. S1† illustrates the UV-vis spectra of gold colloids wherein the SPR spectral values were observed in the regions 588, 562 and 542 nm at different reaction time intervals of 10, 30 and 60 min, which were assigned as g1, g2 and g3, respectively. The spectral result suggests that the SPR peak intensity steadily increases with reaction time. Moreover, the color change arises because of the coherent oscillation of an electron on the surface of the nanoparticles, resulting in SPR with the metal nanoparticles.17 This phenomenon can only be observed in nanoparticles and it is absent in the bulk material.21 In general, Au-NPs exhibit a ruby-red color in aqueous solutions due to excitation of the surface plasmon vibrations.22 The SPR band intensity and bandwidth are influenced by the shape of the particle, dielectric constant of the medium and temperature.23 Therefore, the interaction of light with the Au-NPs leads to polarization of the free conduction electrons with respect to the much heavier ionic core of Au-NPs. Thus, an electron dipolar oscillation is created and a surface plasmon absorption band is found. Chemically synthesized Au-NPs are homogenous in nature, spherical in shape, 40 nm in size, and exhibits a surface plasmon band in the region of 520 nm.24 Herein, the maximum absorption peak is observed in the region of 542 nm at a 60 min reduction time. This peak was assigned to the collective absorption of a ruby reddish color indicating the formation of large Au-NPs. The SPR bands are used to find the dispersibility of metal nanoparticles. In a colloid g1 solution, the peak at 588 nm is characteristic of isotropic nanoparticles, whereas the peak at 542 nm for the g3 solution is characteristic of the plasmon resonance of anisotropic nanoparticles.25–27
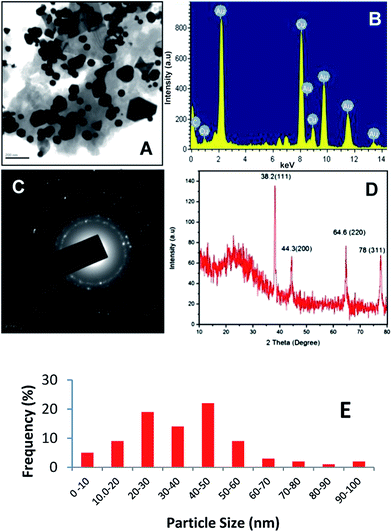
Fig. 1A shows a TEM image of the green synthesized Au-NPs. The TEM images confirm that the metal particles are in the nano-range, hexagonal in shape, with a small number of spherical and few nanoprisms. A histogram of the percent frequency distribution of Au-NPs is shown in Fig. 1E. The nanoprism shaped particles have high surface energy and undergo a shrinking process to reduce the surface energy, resulting in blunt-angled nanoprisms.28 EDX analysis was also used to identify the elemental composition of the gold nanoparticles. Fig 1B shows a strong signal of Au at 2 eV confirming the formation of Au-NPs. Fig. 1C shows the SAED pattern of the synthesized Au-NPs. The bright circular rings correspond to the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) reflections of the fcc gold planes, which clearly indicates that the nanoparticles are crystalline with the same lattice orientation running across the nanoparticles.29
Fig. 1D shows the XRD pattern of the purified Au-NPs. From the XRD pattern confirmed with JCPDS data no. 04-0784, it can be seen that four strong diffraction peaks appear for gold at 38.2°, 44.3°, 64.6°, and 78° 2θ, which were assigned to the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) crystal planes of Au-NPs, respectively, with a lattice parameter of a = 4.078 Å and the space group, Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. No other significant diffraction peaks were detected, indicating that there are no other impurities present with Au-NPs. Furthermore, the spectra indicate a single, sharp and strong XRD peak centered at 38.2° suggesting the strong X-ray scattering centers in the crystalline phase due to the capping agents and stabilizing of the nanoparticles that can be indexed to the (1 1 1) reflection of metallic gold with a fcc structure (JCPDS File no. 04-0784). The weak diffraction peaks at 44.3°, 64.6° and 78° in the pattern agree well with the (2 0 0), (2 2 0) and (3 1 1) reflections, respectively. Generally, peak broadening in the XRD patterns of solids is attributed to the effect of the particle size. Broader peaks indicate a smaller particle size and reflect the effects of the experimental conditions and the nucleation and growth of the crystal nuclei.29,30 Therefore, XRD confirmed that the products are purely Au-NPs with high crystallinity. The average size of the nanoparticles was calculated using the Debye–Scherrer equation to be 32.5 ± 0.25 nm.
m. No other significant diffraction peaks were detected, indicating that there are no other impurities present with Au-NPs. Furthermore, the spectra indicate a single, sharp and strong XRD peak centered at 38.2° suggesting the strong X-ray scattering centers in the crystalline phase due to the capping agents and stabilizing of the nanoparticles that can be indexed to the (1 1 1) reflection of metallic gold with a fcc structure (JCPDS File no. 04-0784). The weak diffraction peaks at 44.3°, 64.6° and 78° in the pattern agree well with the (2 0 0), (2 2 0) and (3 1 1) reflections, respectively. Generally, peak broadening in the XRD patterns of solids is attributed to the effect of the particle size. Broader peaks indicate a smaller particle size and reflect the effects of the experimental conditions and the nucleation and growth of the crystal nuclei.29,30 Therefore, XRD confirmed that the products are purely Au-NPs with high crystallinity. The average size of the nanoparticles was calculated using the Debye–Scherrer equation to be 32.5 ± 0.25 nm.
FTIR spectroscopy is a valuable tool for identifying the involvement of various functional groups in the metal ion interaction during the nanoparticle synthesis process. Fig. S2† shows the FTIR spectra of Justicia glauca leaf powder before (a) and after (b) the encapsulation of Au-NPs. The band positions with corresponding functional groups are given in Table 1. An enormous variety of chemical classes have been found in the species of Justicia, mainly lignans, alkaloids, flavonoids, steroids, and terpenoids, in association with other chemical classes, such as essential oils, vitamins, fatty acids and salicylic acid.18,19 In addition, lignans are the major constituent in the leaf extract, which contains phytochemicals, such as (+)-pinoresinol, (+)-medioresinol, (+)-lariciresinol, (+)-isolariciresinol, (+)-8-methoxy iso lariciresinol, and justiciresinol, and steroids, such as sitosterol-3-0-glucoside, which are responsible for the reduction of metal ions and the efficient stabilization of nanoparticles. Hydroxyl groups (OH−) are very abundant in the chemical constituents. In particular, lignans are rich in hydroxyl groups and might have participated in the gold reduction. These pigments have good reductive properties while AuCl4− is a very strong oxidizing agent and can aid in reduction of Au(III) to Au(0).
AuCl4− + 3R–OH → Au(0) + 3R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + 3H+ + 4Cl− O + 3H+ + 4Cl− |
The above reaction indicates that the reduction of Au(III) to Au(0) occurs through the oxidation of hydroxyl to carbonyl groups.
Electrochemical behavior of Pb2+
To evaluate the electrochemical behavior of Pb2+ at different modified electrodes, differential pulse voltammetry was performed at bare (a) and Au-NPs (b) modified electrodes in 100 μM L−1 Pb2+ containing pH 5 solution. As shown in Fig. 2, the Au-NPs modified electrode showed a well defined cathodic peak at −0.514 V, which is due to the reoxidation of Pb0 to Pb2+.5 Under the same conditions, the unmodified electrode did not show any response to Pb2+, indicating that the bare electrode is unsuitable for the determination of Pb2+. The result further indicates that the good response of Pb2+ is derived from the presence of large active sites of Au-NPs. Therefore, the Au-NPs modified electrode is more suitable for the determination of Pb2+. A schematic representation of the mechanism of Pb2 detection at the Au-NPs modified electrode is shown in Fig. 3. | ||
| Fig. 2 Differential pulse voltammetric response of the bare (a) and Au-NPs (b) modified glassy carbon electrode response to 100 μM L−1 Pb2+ in N2 saturated pH 5 solution. | ||
Optimization of accumulation potential and time
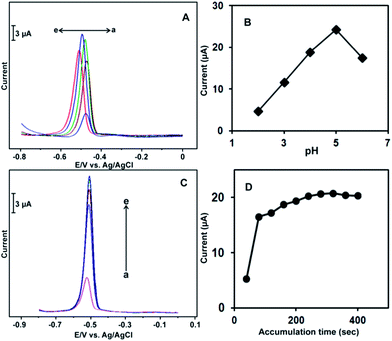
To optimize the experimental conditions for the electrochemical detection of Pb2+ at the Au-NPs modified electrode, the effects of pH, accumulation time and accumulation potential were studied for the response to 100 μM L−1 Pb2+ at the Au-NPs modified electrode. Fig. 4A shows the effects of pH and accumulation on the DPV response of 100 μM L−1 Pb2+. A well-defined DPV response was observed in each pH solution. The response current increased gradually with increasing pH of the solution from pH 2 to pH 6. The highest current response was obtained in the pH 5 solution due to the low concentration of H+ in the solution. The response current decreased when the solution pH was more than 5.0 (Fig. 4B). The current response of the modified electrode to Pb2+ ions decreased below pH 5, which may be due to the disturbance of modified electrode by the large number of hydrogen ions (H+) in solution. In the high pH solutions (more than 7.0), the signal of Pb2+ began to decrease, which may be related to the hydrolysis or valence change in the metal ions. Moreover, the pH 5 (acetate buffer) solution has been widely used as a supporting electrolyte for metal ion detection due to the low background current and good stability. Therefore, pH 5.0 was chosen as the optimal pH for further experiments.Fig. 4C shows the effects of the accumulation time on the DPV of 100 μM L−1 Pb2+ at the Au-NPs modified electrode. The response current increased with increasing deposition time. The highest current response was observed at 220 s; the current response became saturated when the deposition time was more than 220 s (Fig. 4D). Therefore, 220 s was used as the optimal accumulation time. The effect of accumulation potential on the response to 10 μM Pb2+ was also studied by DPV. Fig. S2† shows the effects of the different accumulation potential on the response to 100 μM L−1 Pb2+. The best sensitivity was observed when the deposition potential was swept at −0.8 and −1.2 V. The current intensities of the both accumulation potential (−0.8 and −1.2 V) were identical. The current response decreased when the accumulation potential was approximately −0.8 V. Hence, −0.8 V was chosen as the optimal accumulation potential throughout the experiments.
Determination of Pb2+ at the Au-NPs modified electrode
DPV was employed for the selective determination of Pb2+ at the Au-NPs modified electrode in the pH 5 solution. Fig. 5 shows the DPV response of the Au-NPs modified electrode for Pb2+ at different concentrations. Fig. 5 (inset left side) showed that the Au-NPs modified electrode exhibited a good response to each addition of 5 nM L−1, 50 nM L−1 and 1 μM L−1 of Pb2+. The response current increased linearly with increasing concentration of Pb2+ in the range, 0.005 to 800 μM L−1 Fig. 5 (inset right side). The linear regression equation was found to be y (μA) = 0.234 + 4.07x (μM) with a correlation coefficient of 0.9907. The sensitivity was calculated to be 2.93 μA μM−1 L−1 cm−2 with a detection limit (DL) of 0.07 nM L−1 based on a signal-to-noise ratio of 3. To evaluate the analytical performance of the proposed sensor, the DL and the linear response range were compared with previously reported Pb2+ sensors.37–43 The comparison results are shown in Table 2. The response time (6 s) of the current sensor was comparable to the previously reported Pb2+ sensors, as shown in Table S1.† The response time of the sensor was calculated using amperometry. It was reported that spherical nanoparticles have higher sensitivity and selectivity than non-spherical nanoparticles due to their high stability. However, in our method, most of the nanoparticles were spherical in shape which is why the modified electrode has greater sensitivity and selectivity.44,45 The proposed modified electrode showed a lower DL and a wider linear response range toward Pb2 compared to recently reported Pb2+ sensors.| Modified electrode | LCR (μM L−1) | LOD (nM L−1) | pH | Ref. |
|---|---|---|---|---|
| a Abbreviations: GME – gold microelectrode, DMSA – dimercaptosuccinic acid, IIP – ion imprinted polymeric nanobeads, CPE – carbon paste electrode, L-g-MWCNTS – L-grafted multiwalled carbon nanotubes, NH2–Cu3(BTC)2 – dmino-functionalized metalorganic frameworks, and GCE – glassy carbon electrode. | ||||
| Bi–GME | Up to 6.7 | 12.5 | 12.0 | 37 |
| DMSA–Fe3O4 | Up to 0.05 | 0.5 | 7.6 | 38 |
| IIP–CPE | Up to 1.0 | 0.1 | 4.0 | 39 |
| IIP–CPE | Up to 0.81 | 0.6 | 5.8 | 40 |
| L-g-MWCNTS–CPE | Up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.32 | 6.5 | 41 |
| NH2–Cu3(BTC)2–GCE | Up to 0.5 | 5.0 | 4.5 | 42 |
| MWCNT–GCE | Up to 10 | 4.0 | 4.5 | 43 |
| Au-NPs–GCE | Up to 800 | 0.07 | 5.0 | Present work |
Selectivity of the sensor
To evaluate the selectivity of the Au-NPs modified electrode, the percentage variation of signal of 10 μM L−1 Pb2+ was studied in the presence of a 50 fold concentration of metal cations by DPV. The obtained results are summarized in Fig. 6. Fig. 6 shows that the DPV signal of 10 μM L−1 Pb2+ is unaffected (<2%) in the presence of Co3+, Fe2+, Zn2+ Ca2+, Mg2+, Ni2+ and Cd2+. The signal for Pb2+ changes slightly (<5%) in the presence of Cd2+ and Hg2+. Cu2+ shows serious effect (<8%) on the signal of Pb2+ at the Au-NPs modified electrode. This may be due to the formation of Pb–Cu solid solutions, and similar effects have been reported previously.46 However the effects of other metal cations including Cu2+ on the response of Pb2 were within the acceptable limit, hence the Au-NPs modified electrode is more suitable for the determination of Pb2+. | ||
| Fig. 6 The effect of 50 fold concentrations of interfering metal ions on the percentage variation of signal of 10 μM Pb2 at Au-NPs modified electrode in pH 5 solution. | ||
Real sample analysis
The fabricated Au-NPs modified electrode was applied successfully to the determination of Pb2+ in the river water samples using the standard addition method by DPV. The DPV results are shown in Fig. S4A and B† and the recovery results are summarized in Table 3. The proposed electrode shows good recovery (∼96–98.6%) towards Pb2+ in the river water samples, indicating the good practicality of the proposed electrode.| Sample | Added (μM L−1) | Found (μM L−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| a Abbreviations: RSD – relative standard deviation of three measurements. | ||||
| 2 | 0.5 | 0.48 | 96.0 | 4.2 |
| 3 | 1.2 | 1.19 | 99.2 | 4.7 |
Stability, repeatability and reproducibility
To evaluate the stability of the Au-NPs modified electrode, the response of 50 μM L−1 Pb2+ at the Au-NPs modified electrode was examined periodically for 3 days by DPV. The modified electrode lost about 7.4% of its initial sensitivity after three days. However, no other peak potential shift was observed at the modified electrode, indicating the good stability of the Au-NPs modified electrode. The reproducibility of the sensor was studied using 3 independently prepared Au-NPs modified electrodes towards the detection of 50 μM L−1 Pb2+ by DPV. The DPV results are shown in Fig. S5.† The 3 sensors detected the Pb2+ accurately with a relative standard deviation (RSD) of 2.9%. The fabricated sensor was further employed for the detection of 50 μM L−1 Pb2+ containing 8 solutions and showed good repeatability with a RSD of 3.6. This result indicates the good reproducibility and repeatability of the fabricated sensor.4. Conclusions
In conclusion, a trace level determination of Pb2+ has been demonstrated using green synthesized Au-NPs modified electrode. The synthesized Au-NPs have been characterized by TEM, XRD, EDX, and SAED. The size of the Au-NPs is found as 32.5 ± 0.25. The sensitivity and DL are calculated to be 2.93 μA μM−1 L−1 cm−2 and 0.07 nM L−1, respectively. The proposed sensor shows the high selectivity for Pb2+ in the presence of an excess concentration of other metal ions. Due to the good practicality of the modified electrode, it can be used as a promising electrode material for the selective detection of Pb2+ in environmental samples.Acknowledgements
This project was supported by the National Science Council and the Ministry of Education of Taiwan (Republic of China).Notes and references
- J. Yan and E. M. Indra, Anal. Chem., 2012, 84, 6122 CrossRef CAS PubMed.
- S. M. Zarcero, A. Sanchez, M. Fajardo, I. D. Hierro and I. Sierra, Microchim. Acta, 2010, 169, 57 CrossRef.
- http://www.epa.gov/safewater/contaminants/index.html.
- N. Yildirim, F. Long, M. He, C. Gao, H. C. Shi and A. Z. Gu, Talanta, 2014, 129, 617 CrossRef CAS PubMed.
- Q. Song, M. Li, L. Huang, Q. Wu, Y. Zhou and Y. Wang, Anal. Chim. Acta, 2013, 787, 64 CrossRef CAS PubMed.
- C. W. Lien, Y. C. Chen and H. T. C. Huang, Nanoscale, 2013, 5, 8227 RSC.
- W. Shi, X. Zhang, S. He and Y. Huang, Chem. Commun., 2011, 47, 10785 RSC.
- W. Luo, Y. S. Li, J. Yuan, L. Zhu and Z. Liu, et al., Talanta, 2010, 81, 901 CrossRef CAS PubMed.
- J. W. Lee, H. J. Jeon, H. J. Shin and J. K. Kang, Chem. Commun., 2012, 48, 422 RSC.
- Y. L. Dong, H. G. Zhang, Z. U. Rahman, L. Su and X. J. Chen, et al., Nanoscale, 2012, 4, 3969 RSC.
- C. W. Lien, Y. T. Tseng, C. C. Huang and H. T. Chang, Anal. Chem., 2014, 86, 2065 CrossRef CAS PubMed.
- A. Kumar, S. Mandal, P. R. Selvakannan, R. Pasricha and A. B. Mandale, et al., Langmuir, 2003, 19, 6277 CrossRef CAS.
- K. W. Huang, C. J. Yu and W. L. Tseng, Biosens. Bioelectron., 2010, 25, 984 CrossRef CAS PubMed.
- H. Huang, S. Chen, F. Liu, Q. Zhao and B. Liao, et al., Anal. Chem., 2013, 85, 2312 CrossRef CAS PubMed.
- U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Berlin, Springer, 1995 Search PubMed.
- Y. Li, X. Duan, Y. Qian, L. Yang and H. Liao, J. Colloid Interface Sci., 1999, 209, 347 CrossRef CAS PubMed.
- R. Emmanuel, K. Chelladurai, S. M. Chen, P. Selvakumar, S. Padmavathi and P. Prakash, J. Hazard. Mater., 2014, 279, 117 CrossRef CAS PubMed.
- G. M. Correa and A. F. C. Alcântara, Braz. J. Pharmacog., 2012, 22, 220 CAS.
- N. Fukamiya and K. Lee, J. Nat. Prod., 1986, 49, 348 CrossRef CAS.
- M. Annadhasan, T. Muthukumarasamyvel, V. R. S. Babu and N. Rajendiran, ACS Sustainable Chem. Eng., 2014, 2, 887 CrossRef CAS.
- L. Lina, W. Wang, J. Huang, Q. Li and D. Sun, et al., Chem. Eng. J., 2010, 162, 852 CrossRef PubMed.
- T. K. Joerger, R. Joerger, E. Olsson and G. C. Graqvist, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 13611 CrossRef.
- T. Tanaka, S. Nagao and H. Ogawa, Anal. Sci., 2001, 17, i1081 Search PubMed.
- S. P. Chandran, M. Chaudhary, R. Pasricha, A. Ahmad and M. Sastry, Biotechnol. Prog., 2008, 22, 577 CrossRef PubMed.
- S. L. Smitha, D. Philip and K. G. Gopchandran, Spectrochim. Acta, Part A, 2009, 74, 735 CrossRef CAS PubMed.
- N. Ahmad, S. Sharma, M. K. Alam, V. N. Singh and S. F. Shamsi, et al., Colloids Surf., B, 2010, 81, 81 CrossRef CAS PubMed.
- R. Jenkins and R. L. Snyder, Introduction to X-ray Powder Diffractiometry, John Wiley and Sons, New York, 1996, p. 544 Search PubMed.
- A. N. Mishra, S. Bhadauria, M. S. Gaur, R. Pasricha and B. S. Kushwah, Int. J. Green Nanotechnol., 2010, 1, 118 CrossRef.
- R. Deshpande, M. D. Bedre, S. Basavaraja, B. Sawle and S. Y. Manjunath, et al., Colloids Surf., B, 2010, 79, 235 CrossRef PubMed.
- A. Becheri, M. Durr, P. L. Nostro and P. Baglioni, J. Nanopart. Res., 2008, 10, 679 CrossRef CAS.
- S. S. Shankar, A. Rai, A. Ahmad and M. Sastry, J. Colloid Interface Sci., 2004, 275, 496 CrossRef CAS PubMed.
- J. Y. Song, H. K. Jang and B. S. Kim, Process Biochem., 2009, 44, 1133 CrossRef CAS PubMed.
- R. R. Naik, S. J. Stringer, G. Agarwal, S. Jones and M. O. Stone, Nat. Mater., 2002, 1, 169 CrossRef CAS PubMed.
- A. C. Templeton, J. J. Pietron, R. W. Murray and P. Mulvaney, J. Phys. Chem. B, 2000, 104, 564 CrossRef CAS.
- M. I. Husseiny, M. A. El-Aziz, Y. Badr and M. A. Mahmoud, Spectrochim. Acta, Part A, 2007, 67, 1003 CrossRef CAS PubMed.
- D. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef PubMed.
- M. O. Salles, A. P. R. Souza, J. Naozuka, P. V. Oliveira and M. Bertotti, Electroanalysis, 2009, 21, 1439 CrossRef CAS.
- W. Yantasee, K. Hongsirikarn, C. L. Warner, D. Choi and T. Sangvanich, et al., Analyst, 2008, 133, 348 RSC.
- A. Bahrami, A. B. Seidani, A. Abbaspour and M. Shamsipur, Electrochim. Acta, 2014, 118, 92 CrossRef CAS PubMed.
- T. Alizadeh and S. Amjadi, J. Hazard. Mater., 2011, 190, 451 CrossRef CAS PubMed.
- J. Guo, Y. Chai, R. Yuan, Z. Song and Z. Zou, Sens. Actuators, B, 2011, 155, 639 CrossRef CAS PubMed.
- Y. Wang, H. Ge, Y. Wu, G. Ye, H. Chen and X. Hu, Talanta, 2014, 129, 100 CrossRef CAS PubMed.
- K. Wu, S. Hu, J. Fei and W. Bai, Anal. Chim. Acta, 2003, 129, 215 CrossRef.
- Y. Kim, R. C. Johnson and J. T. Hupp, Nano Lett., 2001, 4, 165 CrossRef.
- K. Yoosaf, B. I. Ipe, C. H. Suresh and K. G. Thomas, J. Phys. Chem. C, 2007, 111, 12839 CAS.
- A. Wanekaya and O. A. Sadik, Electroanal. Chem., 2002, 537, 135 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14988b |
| This journal is © The Royal Society of Chemistry 2015 |