Combined effects of gamma-irradiation and preparation method on antioxidant activity and phenolic composition of Tuberaria lignosa
José Pinelaab,
Amilcar L. Antonioac,
Lillian Barrosad,
João C. M. Barreiraabd,
Ana Maria Carvalhoa,
M. B. P. P. Oliveirab,
Celestino Santos-Buelga d and
Isabel C. F. R. Ferreira*a
d and
Isabel C. F. R. Ferreira*a
aMountain Research Centre (CIMO), ESA, Polytechnic Institute of Bragança, Apartado 1172, 5301-855 Bragança, Portugal. E-mail: iferreira@ipb.pt; Fax: +351-273-325405; Tel: +351-273-303219
bREQUIMTE, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, Rua Jorge Viterbo Ferreira, no. 228, 4050-313, Portugal
cIST/CTN, Campus Tecnológico e Nuclear, Instituto Superior Técnico, Universidade de Lisboa, E.N. 10, 2695-066 Bobadela, Portugal
dGIP-USAL, Facultad de Farmacia, Universidad de Salamanca, Campus Miguel de Unamuno, 37007 Salamanca, Spain
First published on 26th January 2015
Abstract
In this study, the effect of different doses of gamma-irradiation (0, 1, 5 and 10 kGy) on colour, antioxidant activity and phenolic compounds of shade- and freeze-dried samples of Tuberaria lignosa were evaluated and compared. The last two parameters were assessed using decoctions and infusions in order to investigate the influence of the preparation method as well. In general, gamma-irradiation has no influence on colour parameter; changes caused by this technology were only identifiable on the lipid peroxidation inhibition capacity of the shade-dried samples and also on a few phenolic compounds. Differences among preparation method were significant for all assayed parameters, with decoctions being preferable over infusions, as indicated by the higher antioxidant activity and levels of phenolic compounds. Overall, the gamma-irradiation treatment (up to 10 kGy) did not significantly affect the analyzed parameters. Nevertheless, other studies are of interest to evaluate the preservation effectiveness of this technology.
1. Introduction
During the last decades, medicinal and aromatic plants have been extensively studied and found to be excellent sources of bioactive and health-promoting compounds.1,2 Actually, many plant extracts rich in phenolic compounds are used as food complements or can be integrated into cosmetic or pharmaceutical formulations.3,4 For this reason, the industry is very interested in bioactive molecules from natural sources.Based on ethnobotanical surveys conducted in western regions of the Iberian Peninsula, Tuberaria lignosa (Sweet) Samp. (Fam. Cistaceae) arises as one of the most quoted medicinal plants.5,6 After being dried, this plant is used in herbal preparations (infusion and decoction) for treating various diseases and ailments, such as gastrointestinal and hepato-depurative disorders and skin inflammations.6 These local practices are supported by documented biological effects, namely anti-inflammatory and antiulcerogenic (cytoprotective) properties,7 as well as in vitro antioxidant8 and antiviral activities.9 Additionally, the phenolic fraction of this plant, mainly composed of ellagitannins and flavonoids, may be linked to the above mentioned effects.8,9
During the entire production process (from harvesting and drying to packaging and storage), raw medicinal plants are prone to chemical and microbial contaminations and insect infestation, which can lead to spoilage, quality deterioration and consequent economic loss.10,11 Besides being a health hazard to consumers, contaminated medicinal plants can also adversely affect the efficacy and stability of their bioactive compounds, especially during storage,12 and lead to spoilage of pharmaceuticals and food items to which they are added.13 Therefore, an effective and sustainable decontamination process must be followed to ensure the hygienic quality of these products, making them suitable for human consumption and commercialization.
Chemical fumigants have been used to decontaminate plant products, being now prohibited or increasingly restricted in several countries due to health, environmental or occupational safety issues.14 Furthermore, once conventional thermal treatments can damage many plant properties, either chemical or physical,15 new and emerging non-thermal technologies are being investigated and applied. Among them, irradiation processing with gamma-rays is in an exceptional position. This physical method, considered safe and effective by several international authorities (namely FAO, IAEA and WHO),16 has been used for insect disinfestations and parasite inactivation (with low doses up to 1 kGy), reduction of non-spore forming pathogens and spoilage microorganisms (with medium doses from 1 to 10 kGy), and reduction of microorganisms to the point of sterility (achieved at high doses above 10 kGy).13,17 Likewise, the gamma-irradiation treatment provides an alternative way to eliminate pesticide residues from plant products.18 In the European Union, the maximum dose of gamma-irradiation approved to sanitize dried herbs is 10 kGy,19 whereas in USA the maximum is 30 kGy.20
Meanwhile, there is a growing scientific interest in irradiation-induced modifications on antioxidant activity and the compounds responsible for such activity. It is known that during the irradiation process, free radicals and other reactive species are generated due to the interaction with water molecules, capable of breaking chemical bonds and modify various molecules.12 A previous study conducted by our team on T. lignosa showed that it has strong antioxidant activity;8 however the effects of gamma-irradiation on the chemical and physical properties of this plant have never been studied. Therefore, the present study was undertaken to explore the effect of different doses of gamma-irradiation (0, 1, 5 and 10 kGy) on the antioxidant activity, phenolic compounds and colour parameters of shade- and freeze-dried T. lignosa samples. The first two parameters were performed on decoctions and infusions, forms traditionally used for therapeutic applications, in order to investigate the influence of the preparation method as well.
2. Materials and methods
2.1. Standards and reagents
2.2. Samples
Tuberaria lignosa (Sweet) Samp. (synonym of Xolantha tuberaria (L.) Gallego, Munoz Garm & C. Navarro) was collected in the flowering season in Miranda do Douro (Trás-os-Montes, north-eastern Portugal), considering the local medicinal uses as well as healers' and selected consumers' criteria, which are related to particular gathering sites and requirements for safe herbal dosages forms, such as infusion and decoction.The option for wild samples, instead of ones from commercial origin, was supported by a previous work of our research team8 that highlighted wild T. lignosa samples as having higher phenolics content and antioxidant activity than those obtained in a local herbal shop available as dried rosettes of leaves and inflorescences. While the plant material collected in the field is fresh, the commercial one from herbal shops may have been stored for a long period of time or dried differently, which leads to quality loss.
Voucher specimens were deposited in the Herbarium of the Escola Superior Agrária de Bragança, Portugal.
2.3. Samples drying
Tuberaria lignosa flowering aerial parts (e.g., basal leaves, stems and inflorescences) were submitted to two different drying methods: freeze-drying (7750031 Free Zone 4.5, Labconco, Kansas City, MO, USA) immediately after being gathered; and shade-drying, being stored in a dark and dry place in cellophane or paper bags kept at room temperature (∼21 °C and 50% relative humidity) for 30 days, simulating informants' general conditions of traditional plant-use.2.4. Samples irradiation
Freeze- and shade-dried samples were divided into four groups (conveniently packaged in sterilized polyethylene bags): control (non-irradiated, 0 kGy), sample irradiated at 1 kGy, sample irradiated at 5 kGy, and sample irradiated at 10 kGy, where 1, 5 and 10 kGy were the predicted doses.The samples irradiation was performed in a 60Co experimental chamber (Precisa 22, Graviner Manufacturing Company Ltd., UK) with four sources, total activity 177 TBq (4.78 kCi), in January 2014. The estimated dose rate for the irradiation position was obtained with a Fricke dosimeter. During irradiation process, the dose was estimated using Amber Perspex routine dosimeters, following the procedure previously described by Fernandes et al.22 The estimated doses after irradiation were: 0.92 ± 0.01 kGy, 4.63 ± 0.28 kGy and 8.97 ± 0.35 kGy for the freeze-dried samples irradiated at 1, 5 and 10 kGy, respectively; and 1.00 ± 0.04 kGy, 5.07 ± 0.27 kGy and 9.66 ± 0.90 kGy for the shade-dried samples irradiated at 1, 5 and 10 kGy, respectively. The dose rate was 1.9 kGy h−1 and the dose uniformity ratio (Dmax/Dmin) was 1.1 for the freeze- and shade-dried sample irradiated at 1 kGy, and 1.2 for the freeze- and shade-dried sample irradiated at 5 and 10 kGy. For simplicity, in the text, tables and figures, the values 0, 1, 5 and 10 kGy were considered for the doses.
2.5. Colour measurement
A colorimeter (model CR-400; Konica Minolta Sensing, Inc., Japan) with an adapter for granular materials (model CR-A50) was used to measure the colour of the samples. Using illuminant C and the diaphragm opening of 8 mm, the CIE L*, a* and b* colour space values were registered through the computerized system using a colour data software “Spectra Magic Nx” (version CM-S100W 2.03.0006). The instrument was calibrated using the standard white plate before analysis.The colour of the shade- and freeze-dried irradiated and non-irradiated samples was measured in three different points on each set of samples, being considered the average value to determine the colour coordinates L* (lightness ↔ darkness), a* (redness ↔ greenness), and b* (yellowness ↔ blueness).
2.6. Preparation of decoctions and infusions
To prepare decoctions, each sample (1 g) was added to 200 mL of distilled water, heated on a heating plate (VELP Scientific, Usmate, Italy) and boiled for 5 min. The mixture was left to stand at room temperature for 5 min more, and then filtered through Whatman No. 4 paper.To prepare infusions, each sample (1 g) was added to 200 mL of boiling distilled water and left to stand at room temperature for 5 min, and then filtered through Whatman No. 4 paper.
A portion of the obtained decoctions and infusions was frozen and lyophilized (Free Zone 4.5, Labconco, Kansas City, MO, USA) for subsequent analysis of phenolic compounds. The antioxidant properties were evaluated directly on the decoctions/infusions.
2.7. In vitro antioxidant properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/v) brain tissue homogenate which was centrifuged at 3000g for 10 min. An aliquot (0.1 mL) of the supernatant was incubated with the different solution concentrations (0.2 mL) in the presence of FeSO4 (10 μM; 0.1 mL) and ascorbic acid (0.1 mM; 0.1 mL) at 37 °C for 1 h. The reaction was stopped by the addition of trichloroacetic acid (28% w/v, 0.5 mL), followed by thiobarbituric acid (TBA, 2%, w/v, 0.38 mL), and the mixture was then heated at 80 °C for 20 min. After centrifugation at 3000g for 10 min to remove the precipitated protein, the colour intensity of the malondialdehyde (MDA)–TBA complex in the supernatant was measured by its absorbance at 532 nm. The inhibition ratio (%) was calculated using the following formula: Inhibition ratio (%) = [(A − B)/A] × 100, where A and B were the absorbance of the control and the compound solution, respectively.
2 (w/v) brain tissue homogenate which was centrifuged at 3000g for 10 min. An aliquot (0.1 mL) of the supernatant was incubated with the different solution concentrations (0.2 mL) in the presence of FeSO4 (10 μM; 0.1 mL) and ascorbic acid (0.1 mM; 0.1 mL) at 37 °C for 1 h. The reaction was stopped by the addition of trichloroacetic acid (28% w/v, 0.5 mL), followed by thiobarbituric acid (TBA, 2%, w/v, 0.38 mL), and the mixture was then heated at 80 °C for 20 min. After centrifugation at 3000g for 10 min to remove the precipitated protein, the colour intensity of the malondialdehyde (MDA)–TBA complex in the supernatant was measured by its absorbance at 532 nm. The inhibition ratio (%) was calculated using the following formula: Inhibition ratio (%) = [(A − B)/A] × 100, where A and B were the absorbance of the control and the compound solution, respectively.2.8. Phenolic compounds
Each lyophilised decoction/infusion (1 mg) was dissolved in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (80
methanol (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v), filtered through 0.2 μm nylon filters from Whatman, and analysed by HPLC (Hewlett-Packard 1100 chromatograph, Agilent Technologies, Waldbronn, Germany) with a quaternary pump and a diode array detector (DAD) coupled to an HP ChemStation (Rev. A.05.04) data-processing station. A Spherisorb S3 ODS-2 C18 (Waters, Dinslaken, Germany), 3 μm (4.6 mm × 150 mm) column thermostatted at 35 °C was used. The solvents used were: (A) 0.1% formic acid in water, (B) acetonitrile. The elution gradient established was 10% B to 15% B over 5 min, 15–25% B over 5 min, 25–35% B over 10 min, isocratic 50% B for 10 min, and re-equilibration of the column, using a flow rate of 0.5 mL min−1. Double online detection was carried out in the DAD using 280 nm and 370 nm as preferred wavelengths and in a mass spectrometer (MS) connected to the HPLC system via the DAD cell outlet.
20 v/v), filtered through 0.2 μm nylon filters from Whatman, and analysed by HPLC (Hewlett-Packard 1100 chromatograph, Agilent Technologies, Waldbronn, Germany) with a quaternary pump and a diode array detector (DAD) coupled to an HP ChemStation (Rev. A.05.04) data-processing station. A Spherisorb S3 ODS-2 C18 (Waters, Dinslaken, Germany), 3 μm (4.6 mm × 150 mm) column thermostatted at 35 °C was used. The solvents used were: (A) 0.1% formic acid in water, (B) acetonitrile. The elution gradient established was 10% B to 15% B over 5 min, 15–25% B over 5 min, 25–35% B over 10 min, isocratic 50% B for 10 min, and re-equilibration of the column, using a flow rate of 0.5 mL min−1. Double online detection was carried out in the DAD using 280 nm and 370 nm as preferred wavelengths and in a mass spectrometer (MS) connected to the HPLC system via the DAD cell outlet.
MS detection was performed in an API 3200 Qtrap (Applied Biosystems, Darmstadt, Germany) equipped with an ESI source and a triple quadrupole-ion trap mass analyser that was controlled by Analyst 5.1 software. Zero grade air served as the nebuliser gas (30 psi) and turbo gas for solvent drying (400 °C, 40 psi). Nitrogen served as the curtain (20 psi) and collision gas (medium). The quadrupoles were set at unit resolution. The ion spray voltage was set at −4500 V in the negative mode. The MS detector was programmed to perform a series of two consecutive scan modes: enhanced MS (EMS) and enhanced product ion (EPI) analysis. EMS was employed to obtain full scan spectra, to give an overview of all the ions in sample. Settings used were: declustering potential (DP) −450 V, entrance potential (EP) −6 V, collision energy (CE) −10 V. Spectra were recorded in negative ion mode between m/z 100 and 1500. EPI mode was performed in order to obtain the fragmentation pattern of the parent ion(s) of the previous experiment using the following parameters: DP −50 V, EP −6 V, CE −25 V, and collision energy spread (CES) 0 V.
The phenolic compounds present in the decoctions/infusions were characterised according to their UV and mass spectra and retention times, and comparison with authentic standards when available. For quantitative analysis, calibration curves were prepared by injection of known concentrations (2.5–100 μg mL−1) of different standard compounds: apigenin-6-C-glucoside (y = 246.05x − 309.66; R2 = 0.9994); p-coumaric acid (y = 321.99x + 98.308; R2 = 0.9984); ellagic acid (y = 35.695x − 265.7; R2 = 0.9991); gallic acid (y = 556.94x + 738.37; R2 = 0.9968); kaempferol-3-O-glucoside (y = 190.75x − 36.158; R2 = 1); kaempferol-3-O-rutinoside (y = 17 5.02x − 43.877; R2 = 0.9999); luteolin-6-C-glucoside (y = 365.93x + 17.836; R2 = 0.9997); quercetin-3-O-glucoside (y = 316.48x + 2.9142; R2 = 1), and quercetin-3-O-rutinoside (y = 222.79x + 243.11; R2 = 0.9998). The results were expressed in mg per g of lyophilised decoction/infusion.
2.9. Statistical analyses
In all cases, analyses were carried out using three samples separately processed, each of which was further measured three times. Data were expressed as mean ± standard deviation. All statistical tests were performed at a 5% significance level using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., USA).An analysis of variance (ANOVA) with type III sums of squares was performed using the GLM (General Linear Model) procedure of the SPSS software. The dependent variables were analyzed using 2-way ANOVA, with the factors “irradiation dose” (ID) and “preparation method” (PM). When a statistically significant interaction (ID × PM) was detected, the two factors were evaluated simultaneously by the estimated marginal means plots for all levels of each single factor. Alternatively, if no statistical significant interaction was verified, means were compared using Tukey's honestly significant difference (HSD), or other multiple comparison test (t-test).
Principal components analysis (PCA) was applied as pattern recognition unsupervised classification method. The number of dimensions to keep for data analysis was assessed by the respective eigenvalues (which should be greater than one), by the Cronbach's alpha parameter (that must be positive) and also by the total percentage of variance (that should be as high as possible) explained by the number of components selected. The number of plotted dimensions was chosen in order to allow meaningful interpretations.
3. Results and discussion
3.1. Colour assessment
The results for CIE colour L* (lightness), a* (redness) and b* (yellowness) are presented in Table 1. The colour coordinate L* measures the lightness of the sample surface and ranges from black at 0 to white at 100. The chromaticity coordinate a* measures red when positive and green when negative, and chromaticity coordinate b* measures yellow when positive and blue when negative.23 The reported values are given as the mean value of each irradiation dose (ID), including results from shade- or freeze-dried samples, as well as the mean value of each drying method (DM), considering all irradiation doses in each case. The significance of the effect of DM was evaluated using a t-test for equality of means (after checking the equality of variances through a Levene's test), since there were fewer than three groups. The interaction among factors (ID × DM) was never significant (p > 0.05), allowing to compare the effects of each factor per se. As it can be concluded from Table 1, the effect of ID was not significant in any case, indicating that these physical parameters are not strongly affected by gamma-irradiation. On the other hand, the effect of the DM was always significant, showing that samples dried under shade are prone to present lower lightness and redness and higher yellowness. According to the literature, higher L* values and lower a*/b* values are desirable in dried products.24 Therefore, freeze-drying may be indicated as the most suitable DM for T. lignosa samples. Additionally, the lack of significant changes observed in irradiated samples might be an advantageous feature, since the colour parameters are of great importance in food and cosmetics industry.25 In fact, the colour of dried medicinal and aromatic plants is considered as a primary quality criterion23 and is directly related to consumers' appreciation of a product as they tend to associate product colour with its taste, hygienic security, shelf-life and personal satisfaction.26| L* | a* | b* | ||
|---|---|---|---|---|
| a Results are reported as mean values of each irradiation dose (ID), including results from shade- or freeze-dried samples, as well as the mean value of each drying method (DM), considering all irradiation doses in each case. Therefore, SD reflects values in those samples (with different ID or DM), and can be higher than mean values. | ||||
| Irradiation dose (ID) | 0 kGy | 47 ± 5 | 0 ± 3 | 17 ± 3 |
| 1 kGy | 46 ± 6 | 0 ± 3 | 18 ± 3 | |
| 5 kGy | 45 ± 5 | −2 ± 3 | 18 ± 2 | |
| 10 kGy | 43 ± 7 | −1 ± 3 | 18 ± 3 | |
| p-Value (n = 18) | Tukey's test | 0.154 | 0.252 | 0.770 |
| Drying method (DM) | Shade-dried | 41 ± 5 | −2 ± 2 | 19 ± 2 |
| Freeze-dried | 49 ± 4 | 1 ± 2 | 17 ± 3 | |
| p-Value (n = 36) | t-Student's test | <0.001 | <0.001 | <0.001 |
| p-Value (n = 72) | ID × DM | 0.253 | 0.262 | 0.077 |
3.2. Antioxidant activity
The EC50 values obtained for each antioxidant assay are presented in Table 2, both for shade-dried and freeze-dried samples. The reported values are given as the mean value of each irradiation dose (ID), including results from samples submitted to infusion or decoction, as well as the mean value of each preparation method (PM), containing the results for all assayed doses in each case. The significance of the effect of PM was evaluated using a t-test for equality of means (after checking the equality of variances through a Levene's test), since there were fewer than three groups. The interaction among factors (ID × PM) was significant (p < 0.05) in all cases, acting as a source of variability. Thereby, no multiple comparison tests could be performed. However, some conclusions could be drawn after analysing the estimated marginal mean (EMM) plots. For instance, shade-dried (Fig. 1A) and freeze-dried (Fig. 1B) samples, further extracted by decoction, gave greater antioxidant activity than infusion ones. Concerning the effect of ID, the only identifiable tendency was the apparently negative effect of the 5 kGy dose on the lipid peroxidation inhibition capacity in shade-dried samples.| DPPH scavenging activity | Reducing power | Lipid peroxidation inhibition | |||
|---|---|---|---|---|---|
| TBARS formation inhibition | β-Carotene bleaching inhibition | ||||
| a Results are reported as mean values of each irradiation dose (ID), including samples submitted to infusion or decoction, as well as the mean value of each preparation method (PM), considering all irradiation doses in each case. Therefore, SD reflects values in those samples (with different ID or PM), and can be higher than mean values. | |||||
| Shade-dried | |||||
| Irradiation dose (ID) | 0 kGy | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.04 ± 0.02 |
| 1 kGy | 0.2 ± 0.1 | 0.17 ± 0.05 | 0.2 ± 0.1 | 0.02 ± 0.01 | |
| 5 kGy | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.07 ± 0.04 | |
| 10 kGy | 0.3 ± 0.1 | 0.16 ± 0.04 | 0.3 ± 0.1 | 0.03 ± 0.01 | |
| p-Value (n = 18) | Tukey's test | 0.242 | 0.160 | <0.001 | <0.001 |
| Preparation method (PM) | Infusion | 0.39 ± 0.05 | 0.25 ± 0.04 | 0.4 ± 0.1 | 0.05 ± 0.04 |
| Decoction | 0.15 ± 0.01 | 0.11 ± 0.01 | 0.2 ± 0.1 | 0.025 ± 0.002 | |
| p-Value (n = 45) | t-Student's test | <0.001 | <0.001 | <0.001 | <0.001 |
| p-Value (n = 90) | ID × PM | <0.001 | <0.001 | <0.001 | 0.046 |
| Freeze-dried | |||||
| Irradiation dose (ID) | 0 kGy | 0.3 ± 0.1 | 0.16 ± 0.05 | 0.02 ± 0.02 | 0.3 ± 0.1 |
| 1 kGy | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.03 ± 0.02 | 0.2 ± 0.1 | |
| 5 kGy | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.02 ± 0.01 | 0.2 ± 0.1 | |
| 10 kGy | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.03 ± 0.01 | 0.2 ± 0.1 | |
| p-Value (n = 18) | p-value (n = 18) | 0.861 | 0.386 | 0.430 | 0.528 |
| Preparation method (PM) | Infusion | 0.41 ± 0.05 | 0.26 ± 0.04 | 0.04 ± 0.01 | 0.35 ± 0.05 |
| Decoction | 0.15 ± 0.02 | 0.10 ± 0.01 | 0.01 ± 0.01 | 0.11 ± 0.05 | |
| p-Value (n = 45) | t-student's test | <0.001 | <0.001 | <0.001 | <0.001 |
| p-Value (n = 90) | ID × PM | <0.001 | <0.001 | <0.001 | <0.001 |
The interest of decoctions and infusions from shade- and freeze-dried samples of T. lignosa was already highlighted as a source of bioactive compounds and having appreciable antioxidant properties.8 The same work also highlighted wild T. lignosa samples as having higher phenolics content and bioactivity than those obtained in a local herbal shop available as dried rosettes of leaves and inflorescences. That is why wild samples were chosen for this study instead of ones from commercial origin.
Although the antioxidant activities of different medicinal and aromatic plants have already been studied,1–3 nothing has been reported on the effect of gamma-irradiation on the antioxidant activity of T. lignosa. However, some research studies report different effects of the gamma-irradiation treatment on the antioxidant properties of other plant materials. A study conducted by Pereira et al.11 indicated that, in general, the antioxidant properties were increased in borututu (a folk medicine obtained from the African tree Cochlospermum angolense) infusions and methanolic extracts with the irradiation dose of 10 kGy. Carocho et al.27 found that the antioxidant potential of Portuguese chestnuts was increased at 3 kGy. As well, Hussain et al.28 reported a significant decrease in EC50 values (corresponding to a higher antioxidant activity) of sun-dried irradiated (3 kGy) apricots. According to Pérez et al.,29 a 30 kGy dose applied to dry sage and oregano for sanitization did not significantly affect the capacity to inhibit the DPPH radical or the reducing power, nor did it affect the total phenolic content of the methanolic and aqueous extract. Similarly, Mustapha et al.30 observed no significant changes in the free radical scavenging activity of irradiate millet flour up to 5 kGy. In contrast, Kim and Yook31 observed that irradiation of kiwifruit up to 3 kGy had negative effects on vitamin C content and antioxidant activity.
Regarding the use of gamma-irradiation for preservation purposes, its suitability for the hygienization of T. lignosa is unknown; nevertheless some studies support its effectiveness in similar doses for comparable natural matrices, including other dried medicinal and aromatic plants, without affecting their bioactive properties. Chiang et al.32 demonstrated that 2 kGy is sufficient for the inactivation of enterobacteria and 6 kGy for elimination of yeasts and fungi in Polygonum multiflorum Thunb. (an herb used in traditional Chinese medicine), without adversely compromising the total phenols content or the antioxidant potential. Likewise, Kumar et al.33 concluded that an irradiation dose up to 10 kGy is adequate to ensure the microbiological decontamination of Indian herbs retaining their antioxidant properties. Furthermore, in the European Union, the maximum dose of gamma-irradiation approved to sanitize dried herbs is 10 kGy, assuring its decontamination.19
3.3. Phenolic compounds
Table 3a and b shows the quantified amounts of phenolic compounds in non-irradiated and irradiated samples of T. lignosa previously freeze- or shade-dried, respectively. The results are expressed as mean value of each ID for different PM, as well as the mean value of each PM, comprising results for all the assayed ID. In general, despite slight quantitative differences, the phenolic profiles described herein were coherent to those previously characterized in T. lignosa.8 The most abundant compounds were punicalagin isomers (compounds 1 and 3) and punicalagin gallate isomers (compounds 2 and 4) (Fig. 2), which accounted for more than 90% of the quantified phenolic compounds. In fact, T. lignosa was previously reported as an important source of this type of compounds.9 The interaction among factors (ID × PM) was again significant (p < 0.05) in all cases; thus, no multiple comparison tests could be performed. Nevertheless, some observations can be made. In general, shade-dried samples contained lower levels of phenolic compounds than freeze-dried ones. Also a tendency to a decrease in the concentrations of phenolic compounds, especially ellagitannins, was observed in the irradiated samples in relation to non-irradiated ones, which was more accused in the shade-dried samples, although the changes were not statistically significant. Significant differences existed, however, in the levels of compounds depending on the preparation procedure (Fig. 2). With no exception, higher contents of ellagitannins, flavones and flavonols were found in samples extracted by decoction than by infusion, both in shade- and freeze-dried products.| Compound | Irradiation dose (ID) | Tukey's test | Preparation method (PM) | t-Student's test | ID × PM | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 kGy | 1 kGy | 5 kGy | 10 kGy | p-Value (n = 18) | Infusion | Decoction | p-Value (n = 36) | p-Value (n = 72) | |
| a Results are reported as mean values of each irradiation dose (ID), including results from samples submitted to infusion or decoction, as well as the mean value of each preparation method (PM), considering all irradiation doses in each case. Therefore, SD reflects values in those samples (with different ID or PM), and can be higher than mean values. nd – not detected. | |||||||||
| a | |||||||||
| (1) Punicalagin (isomer) | 23 ± 2 | 20 ± 10 | 21 ± 9 | 22 ± 7 | 0.776 | 15 ± 4 | 28 ± 3 | <0.001 | <0.001 |
| (2) Punicalagin gallate (isomer) | 28 ± 11 | 25 ± 14 | 22 ± 12 | 24 ± 8 | 0.561 | 14 ± 3 | 36 ± 3 | <0.001 | <0.001 |
| (3) Punicalagin (isomer) | 47 ± 5 | 37 ± 13 | 43 ± 14 | 43 ± 11 | 0.058 | 32 ± 7 | 53 ± 3 | <0.001 | <0.001 |
| (4) Punicalagin gallate (isomer) | 33 ± 13 | 27 ± 15 | 27 ± 12 | 28 ± 9 | 0.520 | 17 ± 3 | 41 ± 3 | <0.001 | <0.001 |
| (5) Luteolin-6-C-glucose-8-C-glucose | 0.27 ± 0.05 | 0.25 ± 0.05 | 0.28 ± 0.05 | 0.29 ± 0.05 | 0.198 | 0.22 ± 0.02 | 0.33 ± 0.02 | <0.001 | <0.001 |
| (6) 5-O-p-Coumaroylquinic acid | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 1.0 ± 0.4 | <0.001 | 0.8 ± 0.3 | 0.6 ± 0.1 | 0.001 | <0.001 |
| (7) Luteolin-8-C-glucoside | 1.3 ± 0.4 | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.4 ± 0.4 | 0.634 | 0.9 ± 0.1 | 1.9 ± 0.2 | <0.001 | <0.001 |
| (8) Apigenin-8-C-glucoside | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.1 | 0.111 | 1.2 ± 0.1 | 1.4 ± 0.1 | <0.001 | 0.025 |
| (9) Quercetin-3-O-rutinoside | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.349 | 0.16 ± 0.01 | 0.30 ± 0.04 | <0.001 | <0.001 |
| (10) Apigenin-6-C-glucoside | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.1 | 0.003 | 1.2 ± 0.1 | 1.4 ± 0.1 | <0.001 | <0.001 |
| (11) Kaempferol-3-O-rutinoside | 0.37 ± 0.04 | 0.43 ± 0.05 | 0.44 ± 0.05 | 0.41 ± 0.05 | 0.014 | 0.35 ± 0.03 | 0.47 ± 0.05 | <0.001 | <0.001 |
| (12) Luteolin-6-C-glucoside | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.01 ± 0.01 | 0.01 ± 0.01 | <0.001 | nd | 0.02 ± 0.02 | — | — |
| (13) Kaempferol-O-rhamnoside-O-rutinoside | nd | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.002 | nd | 0.2 ± 0.1 | — | — |
| (14) Kaempferol-p-coumaroylglucoside-glutarate | nd | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.001 | nd | 0.2 ± 0.1 | — | — |
| (15) Kaempferol-p-coumaroylglucoside | nd | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.001 | nd | 0.2 ± 0.1 | — | — |
| Phenolic acids | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 1.0 ± 0.4 | <0.001 | 0.8 ± 0.3 | 0.6 ± 0.1 | 0.001 | <0.001 |
| Flavonols | 0.6 ± 0.1 | 1.1 ± 0.5 | 1.1 ± 0.5 | 1.0 ± 0.5 | 0.005 | 0.51 ± 0.02 | 1.4 ± 0.4 | <0.001 | <0.001 |
| Flavones | 4 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 | 0.680 | 3.5 ± 0.2 | 5.0 ± 0.3 | <0.001 | <0.001 |
| Ellagitannins | 130 ± 30 | 109 ± 52 | 114 ± 47 | 118 ± 35 | 0.469 | 78 ± 17 | 158 ± 4 | <0.001 | <0.001 |
| b | |||||||||
| (1) Punicalagin (isomer) | 26 ± 14 | 17 ± 3 | 17 ± 8 | 13 ± 13 | 0.003 | 9 ± 5 | 27 ± 7 | <0.001 | <0.001 |
| (2) Punicalagin gallate (isomer) | 21 ± 13 | 14 ± 4 | 19 ± 10 | 13 ± 13 | 0.086 | 7 ± 4 | 27 ± 6 | <0.001 | <0.001 |
| (3) Punicalagin (isomer) | 50 ± 20 | 32 ± 6 | 33 ± 14 | 24 ± 24 | <0.001 | 19 ± 12 | 50 ± 12 | <0.001 | <0.001 |
| (4) Punicalagin gallate (isomer) | 23 ± 15 | 15 ± 5 | 21 ± 12 | 15 ± 15 | 0.097 | 7 ± 4 | 30 ± 7 | <0.001 | <0.001 |
| (5) Luteolin-6-C-glucose-8-C-glucose | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.09 ± 0.02 | <0.001 | 0.14 ± 0.04 | 0.3 ± 0.1 | <0.001 | <0.001 |
| (6) 5-O-p-Coumaroylquinic acid | 1.1 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.6 ± 0.2 | <0.001 | 0.6 ± 0.2 | 1.0 ± 0.2 | 0.001 | <0.001 |
| (7) Luteolin-8-C-glucoside | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 0.503 | 0.4 ± 0.2 | 2.0 ± 0.3 | <0.001 | <0.001 |
| (8) Apigenin-8-C-glucoside | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.2 | 0.056 | 1.1 ± 0.1 | 1.3 ± 0.1 | <0.001 | <0.001 |
| (9) Quercetin-3-O-rutinoside | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.479 | 0.08 ± 0.03 | 0.32 ± 0.05 | <0.001 | <0.001 |
| (10) Apigenin-6-C-glucoside | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.3 | 0.218 | 1.1 ± 0.1 | 1.5 ± 0.1 | <0.001 | <0.001 |
| (11) Kaempferol-3-O-rutinoside | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.188 | 0.3 ± 0.04 | 0.53 ± 0.05 | <0.001 | <0.001 |
| (12) Luteolin-6-C-glucoside | 0.02 ± 0.02 | 0.002 ± 0.002 | 0.002 ± 0.002 | 0.02 ± 0.02 | <0.001 | nd | 0.02 ± 0.02 | — | — |
| (13) Kaempferol-O-rhamnoside-O-rutinoside | 0.2 ± 0.2 | nd | nd | nd | — | nd | 0.1 ± 0.1 | — | — |
| (14) Kaempferol-p-coumaroylglucoside-glutarate | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.1 | nd | 0.001 | nd | 0.2 ± 0.1 | — | — |
| (15) Kaempferol-p-coumaroylglucoside | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | nd | 0.002 | nd | 0.2 ± 0.1 | — | — |
| Phenolic acids | 1.1 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.6 ± 0.2 | <0.001 | 0.6 ± 0.2 | 1.0 ± 0.2 | 0.001 | <0.001 |
| Flavonols | 1.1 ± 0.5 | 0.8 ± 0.4 | 0.9 ± 0.4 | 0.6 ± 0.3 | 0.018 | 0.4 ± 0.1 | 1.3 ± 0.3 | <0.001 | <0.001 |
| Flavones | 4 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 2 | 0.360 | 2.7 ± 0.4 | 5.2 ± 0.5 | <0.001 | <0.001 |
| Ellagitannins | 121 ± 62 | 78 ± 18 | 90 ± 45 | 65 ± 65 | 0.012 | 42 ± 25 | 135 ± 31 | <0.001 | <0.001 |
 | ||
| Fig. 2 HPLC profile of phenolic compounds in decoction (A) and infusion (B) of T. lignosa freeze-dried samples irradiated with 1 kGy, recorded at 280 nm. | ||
According to Khattak et al.34 the effects of gamma-irradiation on the phenolic content and antioxidant activity would be influenced by plant type and composition, state of the sample (fresh or dry), extraction solvent and procedures, and dose of gamma-irradiation. Furthermore, the irradiation treatment of plant products previously dehydrated under a selected drying method may be a strategy to maintain or improve some chemical or physical parameters.
In general, from the obtained results, it might be concluded that the decoction methodology is preferable to infusion, as indicated by the higher antioxidant activity and levels of phenolic compounds. This finding may be linked to the higher extraction yield achieved with the longer extraction time of decoction compared to infusion. However, local medicinal uses as well as healers' or selected consumers' criteria should be taken into account during the preparation and use of these herbal beverages. Indeed, infusions are commonly used for internal use while decoctions are used for external and topical application. Furthermore, T. lignosa preparations should be avoided during long-term treatments in order to prevent possible side effects or toxicity, which can vary considerably according to the preparation method, doses and physical condition of the individual.6 That is why the folk medicine recommends specific dosages and controlled periods of intake with ritual healing practices.6
The obtained results are in agreement with those of Martins et al.,35,36 who concluded that decoction preparations are preferable over infusions or even hydroalcoholic extracts to achieve higher concentration of flavonoids and total phenolic compounds, as well as greater antioxidant activity, from oregano and thyme plants. Vergara-Salinas et al.37 reported that for extracting phenolics from thyme with water, 100 °C and 5 min are appropriate operating conditions, whereas antioxidant-active non-phenolic compounds were favored at higher temperatures and exposure times. Another recent study, conducted by Martínez-Las Heras et al.,38 concluded that the drying method (including shade- and freeze-drying) and preparation procedures have a great influence on the stability and extractability of bioactive compounds from persimmon leaves. The authors showed that increasing the extraction time (up to 60 min) and temperature (from 70 °C to 90 °C) during water extraction of the herbal beverage increases the concentration of flavonoids and phenolic compounds. Similarly, He et al.39 studied the subcritical water extraction of phenolic compounds from pomegranate seed residues and showed that increasing the same variables (extraction time up to 30 min and temperature up to 220 °C) increases the content of these compounds.
3.4. Principal component analysis (PCA)
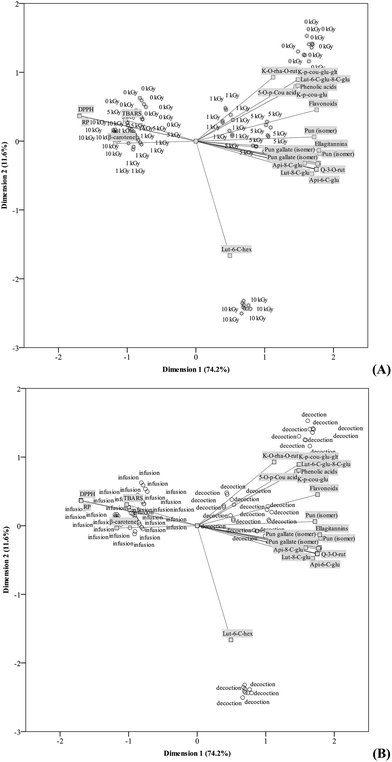
In Sections 3.2. and 3.3., the effects resulting from ID or PM were compared considering antioxidant properties and phenolic composition separately. Despite, some statistically significant changes were observed in both cases, the true effects of the evaluated factors were not completely clear. Accordingly, the results for those parameters were evaluated simultaneously through principal components analysis (PCA). Chromatic parameters were not considered in this analysis since they were measured before the preparation of extracts; furthermore, colour parameters after decoction or infusion of herbs are less relevant.It was intended to verify if differences observed in each evaluated parameter were high enough to correlate with the defined principal components in a way that the geometric distribution of their loadings would lead to the individual clustering of each ID or PM. Regarding shade-dried samples, the first two dimensions (first: Cronbach's α, 17.060; eigenvalue, 0.984; second: Cronbach's α, 2.671; eigenvalue, 0.654) accounted for most of the variance of all quantified variables (74.2% and 11.6%, respectively). Groups corresponding to each gamma-irradiation dose (0 kGy, 1 kGy, 5 kGy and 10 kGy) were not individualized, as it could be hypothesized from Tables 2, 3a and b. In fact, only the group corresponding to those samples irradiated with 10 kGy and prepared by decoction (please confront Fig. 3A and B) were clearly separated from the remaining cases. The other defined groups include objects corresponding to non-irradiated and irradiated samples distributed in a random manner. This mixed grouping did not allow to define which of the assayed parameters better describe each one of applied ID, which might be considered as an indication of the lack of significant effects of gamma-irradiation at the assayed doses (except samples extracted by decoction and further submitted to a 10 kGy ID) on the antioxidant and phenolic profiles of T. lignosa. On the other hand, object points corresponding to each PM were clearly separated, proving that the previously highlighted significant differences were high enough to profile each of these methodologies (Fig. 3B). In an overall analysis, it is clear that samples obtained by decoction have higher amounts of phenolic compounds as also stronger antioxidant activity, as indicated by the diametrically opposed position of their component loadings and the antioxidant activity assays object points.
Concerning freeze-dried samples, the first two dimensions (first: Cronbach's α, 17.383; eigenvalue, 0.985; second: Cronbach's α, 1.739; eigenvalue, 0.444) also accounted for most of the variance of all quantified variables (75.6% and 7.6%, respectively). The obtained outcomes were quite similar, with no separation of object scores according to each of the applied ID (Fig. 4A). Curiously, a small group of objects was individually clustered, as it was verified in shade-dried samples. Nevertheless, in this case, this group corresponded to non-irradiated samples prepared by decoction. This dissimilarity among samples dried using different methodologies is in agreement with the observed significant interaction among factors (ID × PM). As it can be easily deduced from Fig. 4B, object points corresponding to each PM were clearly separated. Once again, infusions showed lower levels in phenolic compounds, as also weaker antioxidant activity.
In general, the preparation method (infusion or decoction) had higher influence in the phenolic profile and antioxidant activity than the irradiation treatment at the applied doses. In addition, CIE colour parameters were also more sensitive to the drying method than irradiation. Differences among infusions and decoctions were significant for all assayed parameters, while changes caused by gamma-irradiation were only significant in TBARS formation inhibition, β-carotene bleaching inhibition and a few phenolic compounds. Besides their effects in individual cases, when all parameters were evaluated together, modifications caused by the preparation method were clearly higher than those observed for gamma-irradiation. As it might be depicted from the PCA plots, object points corresponding to different irradiation doses were grouped arbitrarily, while those corresponding to infusions and decoctions were completely separated. The obtained results indicate that the decoction should be the preferable choice to prepare beverages from this plant, in order to obtain the higher antioxidant activity and phenolic content. Furthermore, the gamma-irradiation treatment (up to 10 kGy), if applied as a preservation technology, will not significantly affect the antioxidant properties of dried T. lignosa samples. Nevertheless, other studies are of interest to evaluate the preservation effectiveness of this technology.
Acknowledgements
The authors are grateful to PRODER research project no. 53514, AROMAP, for financial support of the work and to the Foundation for Science and Technology (FCT, Portugal) for financial support to CIMO (PEst-OE/AGR/UI0690/2011), J. Pinela (SFRH/BD/92994/2013), J.C.M. Barreira (SFRH/BPD/72802/2010) and L. Barros (research contract). The GIP-USAL is financially supported by the Consolider-Ingenio 2010 Programme (FUN-C-FOOD, CSD2007-00063).References
- R. Guimarães, L. Barros, M. Dueñas, R. C. Calhelha, A. M. Carvalho, C. Santos-Buelga, M. J. R. P. Queiroz and I. C. F. R. Ferreira, Food Chem., 2013, 136, 947–954 CrossRef PubMed.
- C. L. Roriz, L. Barros, A. M. Carvalho, C. Santos-Buelga and I. C. F. R. Ferreira, Food Res. Int., 2014, 62, 684–693 CrossRef CAS PubMed.
- M. R. Barroso, L. Barros, M. Dueñas, A. M. Carvalho, C. Santos-Buelga, I. P. Fernandes, M. F. Barreiro and I. C. F. R. Ferreira, Ind. Crops Prod., 2014, 53, 330–336 CrossRef CAS PubMed.
- A. Martins, L. Barros, A. M. Carvalho, C. Santos-Buelga, I. P. Fernandes, M. F. Barreiro and I. C. F. R. Ferreira, Food Funct., 2014, 5, 1091–1100 CAS.
- S. Castroviejo and F. Iberica, Plantas vasculares de la Peninsula Iberica e Islas Baleares, Consejo Superior de Investigaciones Cientificas, Real Jardin Botanico, Madrid, 2005, vol. III Search PubMed.
- A. M. Carvalho, Persistence of Wild Food and Wild Medicinal Plant Knowledge in a North-Eastern Region of Portugal, in Ethnobotany in the New Europe: People, Health and Wild Plant Resources, Berghahn Books, ed. M. Pardo de Santayana, A. Pieroni and R. Puri, Oxford, UK, 2013, pp. 147–171 Search PubMed.
- S. Martin-Aragón, J. Benedi and A. Villar, Int. J. Pharmacogn., 1994, 32, 27–32 CrossRef.
- J. Pinela, L. Barros, M. Dueñas, A. M. Carvalho, C. Santos-Buelga and I. C. F. R. Ferreira, Food Chem., 2012, 135, 1028–1035 CrossRef CAS PubMed.
- L. M. Bedoya, M. J. Abad, S. Sanchez-Palomino, J. Alcami and P. Bermejo, Phytomedicine, 2010, 17, 69–74 CrossRef CAS PubMed.
- B. Darfour, S. Agbenyegah, D. O. Ofosu, A. A. Okyere and I. K. Asare, Radiat. Phys. Chem., 2014, 102, 153–158 CrossRef CAS PubMed.
- C. Pereira, R. C. Calhelha, A. L. Antonio, M. J. R. P. Queiroz, L. Barros and I. C. F. R. Ferreira, Innovative Food Sci. Emerging Technol., 2014, 26, 271–277 CrossRef CAS PubMed.
- F. Aouidi, H. Ayari, H. Ferhi, S. Roussos and M. Hamdi, Food Chem., 2011, 127, 1105–1113 CrossRef CAS PubMed.
- H. Machhour, I. El Hadrami, B. Imziln, M. Mouhib and M. Mahrouz, Radiat. Phys. Chem., 2011, 80, 604–607 CrossRef CAS PubMed.
- UNEP, Montreal Protocol on Substances that Deplete the Ozone Layer, Report of the Methyl Bromide Technical Options Committee. Nairobi, Kenya, United Nations Environment Programme, 2006.
- A. Rawson, A. Patras, B. K. Tiwari, F. Noci, T. Koutchma and N. Brunton, Food Res. Int., 2011, 44, 1875–1887 CrossRef CAS PubMed.
- WHO, High-dose irradiation: Wholesomeness of food irradiated with doses above 10 kGy, Report of a joint FAO/IAEA/WHO study group, WHO technical Report Series 890, World Health Organization, Geneva, Switzerland, 1999 Search PubMed.
- ICGFI, Facts about Food Irradiation: A series of Fact Sheets from the International Consultative Group on Food Irradiation, International Consultative Group on Food Irradiation, Vienna, Austria, 1999 Search PubMed.
- H.-W. Wen, M.-F. Hsieh, Y.-T. Wang, H.-P. Chung, P.-C. Hsieh, I.-H. Lin and F.-I. Chou, J. Hazard. Mater., 2010, 176, 280–287 CrossRef CAS PubMed.
- Directive 1999/3/EC, Off. J. Eur. Communit., 1999, 66, 24–25.
- FDA, 21CFR 179, Irradiation in the production, processing and handling of food, Revised as of April 1, Federal Registration, 2012.
- ASTM, Practice for using the Fricke reference standard dosimetry system, ASTM E1026. Annual book of ASTM standards, 12.02, American Society for Testing and Materials, Philadelphia, PA, 1992 Search PubMed.
- Â. Fernandes, A. L. Antonio, J. C. M. Barreira, M. L. Botelho, M. B. P. P. Oliveira, A. Martins and I. C. F. R. Ferreira, Food Bioprocess Technol., 2013, 6, 2895–2903 CrossRef.
- D. Argyropoulos and J. Müller, J. Appl. Res. Med. Arom. Plant., 2014, 1, 15–22 Search PubMed.
- M. Rahimmalek and S. A. H. Goli, Ind. Crops Prod., 2013, 42, 613–619 CrossRef CAS PubMed.
- C. Jo, J. H. Son, M. G. Shin and M.-W. Byun, Radiat. Phys. Chem., 2003, 67, 143–148 CrossRef CAS.
- B. Sturm, A.-M. N. Vega and W. C. Hofacker, Appl. Therm. Eng., 2014, 62, 455–460 CrossRef PubMed.
- M. Carocho, A. L. Antonio, L. Barros, A. Bento, M. L. Botelho, I. Kaluska and I. C. F. R. Ferreira, Food Chem. Toxicol., 2012, 50, 3452–3455 CrossRef CAS PubMed.
- P. R. Hussain, S. Chatterjee, P. S. Variyar, A. Sharma, M. A. Dar, A. M. Wani and J. Food Compos, Analyst, 2013, 30, 59–66 CAS.
- M. B. Pérez, S. A. Banek and C. A. Croci, Food Chem., 2011, 126, 121–126 CrossRef PubMed.
- M. B. Mustapha, M. Bousselmi, T. Jerbi, N. B. Bettaïeb and S. Fattouch, Food Chem., 2014, 154, 230–237 CrossRef PubMed.
- K.-H. Kim and H.-S. Yook, Radiat. Phys. Chem., 2009, 78, 414–421 CrossRef CAS PubMed.
- Y.-C. Chiang, G.-J. Huang, Y.-L. Ho, P.-C. Hsieh, H.-P. Chung, F.-I. Chou and Y.-S. Chang, Process Biochem., 2011, 46, 777–782 CrossRef CAS PubMed.
- S. Kumar, S. Gautam, S. Powar and A. Sharma, Food Chem., 2010, 119, 328–335 CrossRef CAS PubMed.
- K. F. Khattak, T. J. Simpson and Ihasnullah, Food Chem., 2008, 110, 967–972 CrossRef CAS PubMed.
- N. Martins, L. Barros, C. Santos-Buelga, M. Henriques, S. Silva and I. C. F. R. Ferreira, Food Chem., 2014, 158, 73–80 CrossRef CAS PubMed.
- N. Martins, L. Barros, C. Santos-Buelga, S. Silva, M. Henriques and I. C. F. R. Ferreira, Food Chem., 2015, 167, 131–137 CrossRef CAS PubMed.
- J. R. Vergara-Salinas, J. Pérez-Jiménez, J. L. Torres, E. Agosin and J. R. Peérez-Correa, J. Agric. Food Chem., 2012, 60, 10920–10929 CrossRef CAS PubMed.
- R. Martínez-Las Heras, A. Heredia, M. L. Castelló and A. Andres, Food Biosci., 2014, 6, 1–8 CrossRef PubMed.
- L. He, X. Zhang, H. Xu, C. Xu, F. Yuan, Z. Knez, Z. Novak and Y. Gao, Food Bioprod. Process., 2012, 90, 215–223 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |