Soluble bipolar star-shaped molecule as highly stable and efficient blue light emitter
Abstract
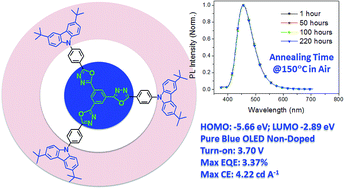
A star-shaped blue small-molecule 1,3,5-tris(5-(4-(3,6-di-tert-butylcarbazol-9-yl)phenyl)-1,3,4-oxadiazol-2-yl)benzene (S-Cz-OXD) with donor–π–acceptor (D–π–A) structure was designed and synthesized by incorporating three (3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl arms into an tris(1,3,4-oxadiazole)phenylene core. It gives pure blue photoluminescence emission at 424 nm with high quantum efficiency (Φf) (93%) in dilute toluene and has good solubility in common solvents. This star-shaped molecule also demonstrates enhanced Φf (72%) in the film state. Moreover, the spectral stability of S-Cz-OXD is also excellent due to its low lying HOMO level and absence of fluorene moiety. The PL spectrum of S-Cz-OXD remains virtually unchanged even after annealing at 150 °C for 220 hours in air. The solution-processed non-doped electroluminescent device shows a low turn-on voltage of 3.7 V although there exists a big energy barrier due to the bipolar design. The device emits stable pure blue light, CIE (Commission Internationale de L'Eclairage) coordinates of (0.16, 0.16) with high current efficiency (CE) and external quantum efficiency (EQE) of 4.22 cd A−1 and 3.37% respectively. Mechanistic discussion is also carried out in relation to the molecular structure.

 Please wait while we load your content...
Please wait while we load your content...