Preparation and electrochemical performance of Mo6V9O40 nanorods as cathode materials for Li batteries†
Abstract
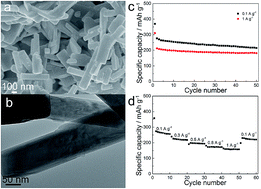
Mo6V9O40 nanorods were prepared through a sol–gel route followed by short-time calcination, and tested as cathode materials for lithium batteries. The Mo6V9O40 nanorods demonstrated good cyclic stability and rate performance at deep discharge (discharged to 1.5 V), which were attributed to the enhanced structural flexibility and the 1D structure.


 Please wait while we load your content...
Please wait while we load your content...