A novel bifunctional molecularly imprinted polymer for determination of Congo red in food
Abstract
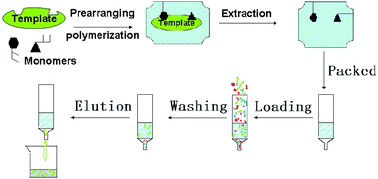
A novel bifunctional molecularly imprinted polymer was synthesized in an aqueous system with Congo red as template, beta-cyclodextrin-maleic anhydride (β-CD-MA) and [2-(methacryloyloxy) ethyl] trimethylammonium chloride (DMC) as co-functional monomers and N,N-methylenebisacryl amide (MBA) as cross-linking agent, respectively. MIP was used as selective sorbent for the solid-phase extraction (SPE) of Congo red from food. And the elution was determined by HPLC. The mean recovery was 84.2–105.2%, the RSD ≤ 1.2%, the repeatability was 1.1–3.8%. The LOD and LOQ were 0.07 and 0.23 μg kg−1, respectively. The evaluation of validation data verified that the MIP has high selectivity and recovery, and it can be applied for the selective extraction and determination of Congo red in food.

 Please wait while we load your content...
Please wait while we load your content...