Ruthenium complex immobilized on poly(4-vinylpyridine)-functionalized carbon-nanotube for selective aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran†
Jinzhu Chen*a,
Jiawei Zhongac,
Yuanyuan Guoa and
Limin Chenb
aCAS Key Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, PR China. E-mail: chenjz@ms.giec.ac.cn; Fax: +86-20-3722-3380; Tel: +86-20-3722-3380
bCollege of Environment and Energy, South China University of Technology, Guangzhou 510006, PR China
cUniversity of Chinese Academy of Sciences, Beijing 100049, PR China
First published on 12th December 2014
Abstract
Polymer/carbon composite material of poly(4-vinylpyridine)-functionalized carbon-nanotube (PVP/CNT) was prepared by in situ polymerization of 4-vinylpyridine monomer in the presence of CNT suspension. Raman spectra analysis confirmed the almost unchanged graphitized surfaces of CNT moiety in the PVP/CNT after the covalent functionalization of pristine CNT with PVP. Catalyst made of ruthenium complex immobilized on PVP/CNT (Ru-PVP/CNT) was fully characterized by ICP-OES, TG-DTA, FT-IR, Raman, XRD, UV-vis, BET, TEM, XPS and H2-TPR. Moreover, Ru-PVP/CNT shows excellent catalytic performance towards the selective oxidation of biomass-based 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF) with molecular oxygen as oxidant. The reaction parameters such as the reaction temperature, reaction time, solvent, catalyst amount, oxidant, and oxygen pressure were systematically investigated for this important biomass-related transformation. Under the optimal condition, a DFF yield of 94% with a full HMF conversion were obtained by using Ru-PVP/CNT in N,N-dimethylformamide (DMF) under 2.0 MPa O2 at 120 °C.
1. Introduction
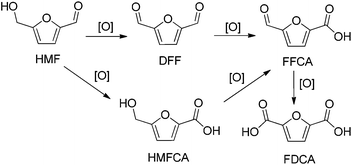
With the rapid depletion of fossil fuels and the aggravation of global warming, much effort has been devoted to the production of fuels and chemicals from renewable resources. As the only carbon-containing renewable resource, biomass serves as an alternative feedstock to provide fuels and chemicals.1,25-Hydroxymethylfurfural (HMF), obtained from acid-promoted dehydration of C6 based carbohydrate, has been identified as a key platform chemical compound which can be transformed to a variety of valuable chemicals and fuels.3,4 In particular, the selective oxidation of HMF to 2,5-diformylfuran (DFF) has attracted considerable interests in recent decades, due to the applications of DFF in monomer, pharmaceuticals,5 fungicides,6 furan–urea resins7 as well as heterocyclic ligand.8 However, the selective oxidation of HMF is influenced by various parameters such as reaction temperature, reaction time, solvent, oxidants etc. In addition, some byproducts such as 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), 5-formyl-2-furancarboxylic acid (FFCA), 2,5-furandicarboxylic acid (FDCA) can be produced during the process (Scheme 1). Therefore, it is still a challenging task for the selective oxidation of HMF to DFF.
The selective oxidation of HMF to DFF have been reported by using conventional oxidants such as NaOCl,9 BaMnO4,10 Pb(OAc)4–pyridine,11 K2Cr2O7–DMSO, trimethylammonium chlorochromate (TMACC),12 oxalylchloride (OC), pyridinium chlorochromate (PCC) and 2,2,6,6-tetramethylpiperidine-1-oxide (TEMPO).13–15 Nevertheless, the requirement of stoichiometric oxidants and the release of hazardous wastes are unavoidable for these methods. Therefore, selective oxidation of HMF to DFF with environment friendly molecular oxygen as oxidant satisfied the requirement of green chemistry.
To date, homogeneous metal/bromide systems (Co/Mn/Br, Co/Mn/Zr/Br)16 and heterogeneous transition metal-based catalysts such as ruthenium,17–21 vanadium,22–31 manganese,9,32,33 copper23,26 and silver34 have been developed for the production of DFF from HMF or directly from carbohydrate.35–40 Recently, our team achieved one-step approach to DFF directly from fructose by using acidic cesium salts of molybdovanadophosphoric heteropolyacids (CsMVP-HPAs)39 as well as proton- and vanadium-containing graphitic carbon nitride [V-g-C3N4(H+)].40 Among the developed catalyst systems, ruthenium-based catalyst is proved to be more active and selective to this important biomass-related transformation of HMF to DFF. For example, Ebitani and coworkers obtained DFF from HMF with yield of 92% over hydrotalcite-supported ruthenium catalyst [Ru(OH)x/HT] in N,N-dimethylformamide (DMF) using molecular oxygen as oxidant.38 Antonyraj et al. achieved DFF from HMF with yield of 97% on RuCl3/Al2O3 at 130 °C with molecular oxygen.19 Zhang and team members obtained DFF yield of 86.4% with Fe3O4@SiO2–NH2–Ru(III) at 120 °C. It was found that Ru3+ ions from RuCl3 aqueous solution can be effectively immobilized by amino group of Fe3O4@SiO2–NH2.18 In addition, Corma et al. obtained a DFF yield of 82% on immobilized vanadyl pyridine complexes at 130 °C. It was observed that vanadyl groups can be fully coordinated with pyridine ligand.29 Therefore, pyridine–Ru(III) complex was investigated as catalyst for selective aerobic oxidation of HMF to DFF in this research.
Currently, commercially available carbon materials such as carbon nanotubes (CNT) have attracted ever-increasing attention due to their high surface area, tunable surface properties, superior mechanical strength etc. However, the lack of solubility and the difficult manipulation of CNT in most solvents have limited their use. Therefore, CNT generally needs to undergo chemical functionalization to enhance solubility in various solvents and to produce novel hybrid materials for practical applications.41 By comparison, there is a large potential for the development and application of organic polymer with tailored physical and chemical properties in both selective catalysis and functional material science, owing to the possibility of employing the vast range of organic transformations developed by synthetic organic chemists.42,43 Therefore, to combine the advantages of polymer and CNT, much effort has been put in polymer/CNT composite material. For instance, our group recently developed poly(p-styrenesulfonic acid)-functionalized CNT (CNT–PSSA) as solid acid for effective conversion of biomass-based fructose to HMF and alkyl levulinate.44
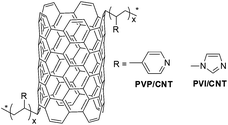
In recent decades, transition metal complex with nitrogen-containing polymer as ligand or immobilizer has attracted great interests for catalysis purpose. Among the developed nitrogen-containing polymers, due to the strong affinity of the pyridyl group with transition metal ions, poly(4-vinylpyridine) (PVP)-immobilized transition metal complexes45–47 have particularly been used in various applications such as chemoselective protection of aldehydes,48 selective solid phase extraction,49 hydrogenation and hydroformylation,50 water gas shift reaction,51 reduction of nitrobenzene.51 Recently, our team investigated a series of nitrogen-containing polymer, including PVP- and poly(1-vinylimidazole) (PVI)-functionalized CNT (Fig. 1). Moreover, the catalysts, made of palladium supported on these polymer/CNT composite materials, showed excellent selectivity towards hydrogenation of phenol and derivatives. Our research results further indicate that the phenol conversion is related to the conductive property of polymer/CNT; whereas, the cyclohexanone selectivity is contributed to the nitrogen-containing nature of polymer/CNT.42,43
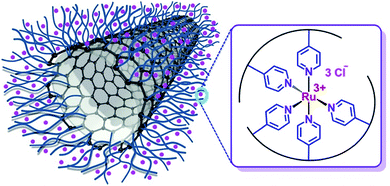
To explore the application of composite material PVP/CNT, ruthenium complex was immobilized on the PVP/CNT (Ru-PVP/CNT) through the formation of coordinative bond N: → Ru3+ (Fig. 2). Raman spectra analysis confirmed the almost unchanged graphitized surfaces of CNT moieties in both PVP/CNT and Ru-PVP/CNT after the functionalization of pristine CNT. Moreover, the coordinative bond of N: → Ru3+ in Ru-PVP/CNT was confirmed by Ultraviolet visible (UV-vis) spectrum and X-ray photoelectron spectroscopy (XPS) analysis. The synthesized catalyst Ru-PVP/CNT shows excellent catalytic performance towards the selective oxidation of HMF to DFF with molecular oxygen as oxidant (Scheme 1). Under the optimal condition, a DFF yield of 94% with full HMF conversion were obtained by using Ru-PVP/CNT in DMF under 2.0 MPa O2 at 120 °C.
2. Results and discussion
2.1 PVP/CNT and Ru-PVP/CNT
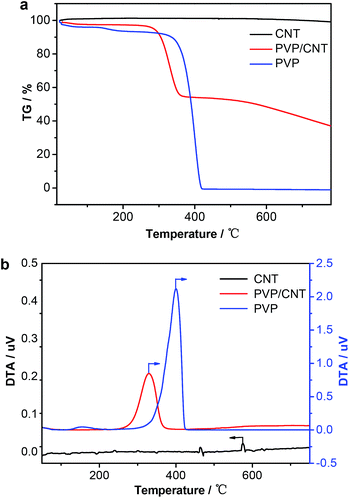
PVP/CNT composite material was prepared by polymerization of 4-vinylpyridine monomer with azobisisobutyronitrile (AIBN) as a radical initiator in the presence of CNT suspension. According to literature,41,43,52 the CNT is functionalized by poly(4-vinylpyridine) through covalent chemical grafting in PVP/CNT. The catalyst of Ru-PVP/CNT was synthesized by treating PVP/CNT with aqueous solution of RuCl3, followed with filtration and drying under vacuum. The inductively coupled plasma-optical emission spectroscopy (ICP-OES) analysis of the Ru-PVP/CNT showed that 2.2 wt% of Ru was immobilized on PVP/CNT.The thermal stabilities of CNT, PVP and PVP/CNT were investigated by thermal gravimetric-differential thermal analysis (TG-DTA). As shown in Fig. 3, CNT shows no significant weigh lose before 800 °C. By contrast, PVP shows the weight loss of 2.4% at 100 °C, which is attributed to the loss of residual moisture associated with PVP. In addition, PVP further shows weight loss of 97.6% at 418 °C as supported with a sharp DTA at 405 °C. As for PVP/CNT, beside the weight loss of 2.5% at 100 °C attributed to the loss of residual moisture, the main decomposition started at 290 °C with the weight loss of 26.3% which was supported by DTA at 330 °C. The similar TG-DTA results were observed in fullerene[60]–polyvinylpyridine composites.53 TG-DTA of PVP/CNT thus confirmed that CNT was successfully functionalized by polymer PVP.
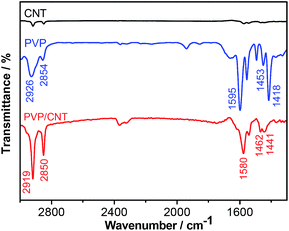
The Fourier transform infrared (FT-IR) spectra of PVP and PVP/CNT are compared in Fig. 4. As we have investigated previously, the FT-IR spectrum of pristine CNT is practically featureless with extremely low infrared absorption intensities.42,44 For PVP/CNT, the IR bands at 2919 and 2850 cm−1 were assigned to asymmetric and symmetric vibration absorptions, respectively, for C–H band of the aliphatic CH2 in vinyl chain. In the case of PVP, the corresponding bands are observed at 2926 and 2854 cm−1, respectively. In addition, the absorption bands of PVP/CNT around 1580 cm−1 are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of pyridine ring. The absorption bands at 1462 and 1441 cm−1 are attributed to C
C stretching vibration of pyridine ring. The absorption bands at 1462 and 1441 cm−1 are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of pyridine ring in PVP/CNT, which are in accordance with the reported observance.54 The spectrum shifts between PVP and PVP/CNT are attributed to the strong π–π interaction between PVP and CNT moiety in the PVP/CNT composite.42,43 Therefore, FT-IR results further verify the existence of PVP chain segments in the as-prepared PVP/CNT composite materials.
N stretching vibration of pyridine ring in PVP/CNT, which are in accordance with the reported observance.54 The spectrum shifts between PVP and PVP/CNT are attributed to the strong π–π interaction between PVP and CNT moiety in the PVP/CNT composite.42,43 Therefore, FT-IR results further verify the existence of PVP chain segments in the as-prepared PVP/CNT composite materials.
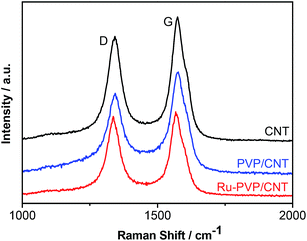
Raman spectroscopy is used to determine the changes of defect density in the CNT moiety of PVP/CNT and Ru-PVP/CNT after the polymerization and immobilization process (Fig. 5). The peak near 1576 cm−1 (G-band) corresponds to the E2g mode of graphite. Whereas, the peak around 1342 cm−1 (D-band) is associated with the vibrations of carbon atoms in the disordered graphite structure, i.e., the defect sites. Generally, the intensity ratio of the D-band to G-band (ID/IG) is used to determine the defect density of CNT. The ID/IG is 0.85 for the pristine CNT and then slightly increases to 0.88 for PVP/CNT and to 0.89 for Ru-PVP/CNT. Therefore, Raman spectra analysis indicates that graphitized surfaces of CNT moieties in both PVP/CNT and Ru-PVP/CNT were almost unchanged after the covalent functionalization of CNT with polymer PVP to give PVP/CNT and the immobilization of Ru complex on PVP/CNT to afford Ru-PVP/CNT, respectively.
The UV-vis spectra of PVP/CNT and Ru-PVP/CNT are compared in Fig. S1 (ESI†). For PVP/CNT, the bands at 207 and 255 nm are attributed to the absorptions of π → π* and n → π* orbits of PVP, respectively. In the case of Ru-PVP/CNT, the corresponding absorption band shift to 210 and 258 nm, respectively. Therefore, the presence of Ru3+ ions in Ru-PVP/CNT lead to red shifts of PVP/CNT in both bands, which is attributed to the formation of coordination bonds between pyridyl ligand in PVP and Ru3+ ions in the Ru-PVP/CNT.
The Brunauer–Emmett–Teller (BET) surface areas and the pore sizes of PVP/CNT and Ru-PVP/CNT were calculated with BET and Barrett–Joyner–Halenda (BJH) methods. As determined from the nitrogen adsorption/desorption isotherm in Fig. S2 (ESI†), the average pore diameter and total pore volume for PVP/CNT are 16.5 nm and 0.35 cm3 g−1, respectively. By comparison, the average pore diameter and total pore volume for Ru-PVP/CNT decrease to 14.6 nm and 0.33 cm3 g−1, respectively. However, BET surface areas of PVP/CNT and Ru-PVP/CNT are 84.8 and 89.4 m2 g−1. The slightly increased surface area can be attributed to the formation of new pore in the matrix of PVP/CNT.42
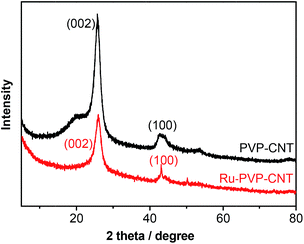
The powder X-ray diffraction (XRD) patterns of PVP/CNT and Ru-PVP/CNT show no distinct difference (Fig. 6), though a slight attenuation of the diffraction peaks at 26°, indicating that the immobilization process does not greatly damage the crystallinity structure of PVP/CNT. The diffraction peaks of both PVP/CNT and Ru-PVP/CNT located at a 2θ value of about 26° and 43° were assigned to the characteristic peaks of the (002) and (100) packing of graphitic CNT, respectively.42,43 Moreover, the distinct reflections for Ru(0) and RuO2 were unobserved in XRD results,55 which proves the unchanged valence state of Ru(III) during the catalyst preparation process.18,55
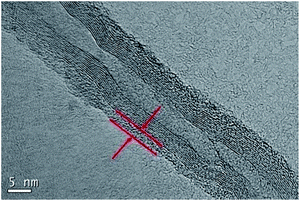
The surface morphologies of the CNT and PVP/CNT were further observed by transmission electron microscopy (TEM). The outside diameter of pristine CNT falls into the rage of 10 to 20 nm with the CNT length around 10–30 um (Fig. S3, ESI†). In contrast, PVP/CNT shows a thin polymer coating of 2–4 nm over CNT (Fig. 7). Thus, TEM analysis of PVP/CNT effectively confirmed the functionalization of CNT with polymer PVP.
The surface composition of PVP/CNT and Ru-PVP/CNT were investigated with X-ray photoelectron spectroscopy (XPS). Peaks corresponding to carbon, oxygen, nitrogen, ruthenium and chlorine are presented in the survey scan (Fig. 8a). Corresponding O 1s signals were possibly arose from surface impurities.56 For Ru-PVP/CNT, there is an overlap between Ru 3d and C 1s peak at around 282.5 eV.18,56 The presence of chlorine peak indicated that Cl− ion acts as the counter ion of Ru3+ ion.45 The binding energy at 461.8 and 484.2 eV are assigned to Ru 3p3/2 and Ru 3p1/2, respectively, which demonstrates that the oxidation state of the ruthenium species is Ru3+ (Fig. 8b).18,57 The N 1s binding energies are 397.0 and 397.4 eV for PVP/CNT and Ru-PVP/CNT, respectively (Fig. 8c). The observed shift in N 1s binding energies indicates that coordination bonds are presumably formed between nitrogen atom of pyridyl ligand and Ru3+ ion in Ru-PVP/CNT.
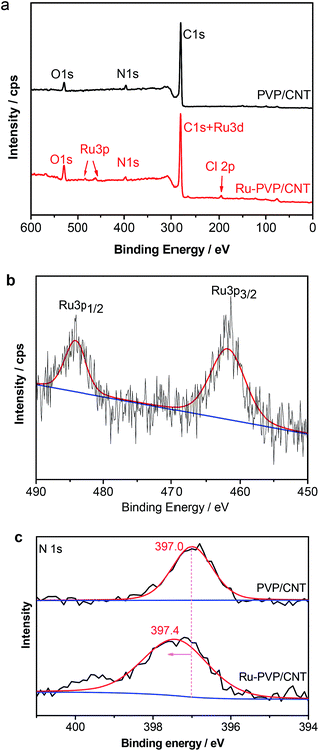 | ||
| Fig. 8 (a) XPS scan survey for PVP/CNT and Ru-PVP/CNT, (b) Ru 3p XPS spectra of Ru-PVP/CNT, (c) N 1s XPS spectra of PVP/CNT and Ru-PVP/CNT. | ||
The temperature-programmed reduction (H2-TPR) profile for Ru-PVP/CNT is provided in Fig. S4 (ESI†). Two reduction peaks take place around 173 and 540 °C for Ru-PVP/CNT, the peak at 173 °C results from the reduction of Ru complex; whereas, hydrogen consumption observed at 540 °C is attributed to the methanation of PVP/CNT.58 Therefore, the H2-TPR profile effectively testifies that Ru species is in the form of Ru3+ rather than Ru0 in Ru-PVP/CNT.
2.2 Selective aerobic oxidation of HMF over Ru-PVP/CNT
The prepared Ru-PVP/CNT was investigated as potential catalyst for selective aerobic oxidation of HMF to DFF. The reaction is typically carried out in DMF under 2.0 MPa O2. Fig. 9 shows the effects of reaction temperature and time on the aerobic oxidation of HMF to DFF. Both the HMF conversion and DFF yield increased with reaction time at all temperatures investigated. The yield of DFF increased slowly to 67% after 12 h at 100 °C; whereas, it increased quickly to 94% after 12 h at 120 °C, confirming that increasing the reaction temperature promotes the aerobic oxidation of HMF to DFF. The result was consistent with the previous reports.17–19,32,34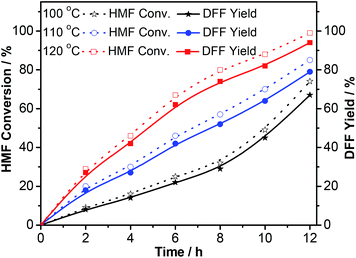 | ||
| Fig. 9 The influences of reaction temperature and time on the aerobic oxidation of HMF to DFF. Reaction conditions: HMF (63 mg, 0.5 mmol), Ru-PVP/CNT (60 mg, Ru 2.2 wt%), DMF (5 mL), O2 (2.0 MPa). | ||
It is widely accepted that the nature of solvent have great impacts on the conversion rate of HMF and selectivity to DFF. Therefore, various solvents such as high boiling and polar solvents [DMF and dimethylsulfoxide (DMSO)], low boiling and polar solvents [isopropyl alcohol (IPA), ethanol (EtOH), acetonitrile (MeCN), 1,4-dioxane and water] and non-polar solvent (toluene) are investigated so as to study the solvent effect on the aerobic oxidation of HMF. Among all the solvents tested, DMF showed HMF conversion of >99% with DFF selectivity of 95% (Table 1, Entry 1), which may be ascribed to the high solubility of oxygen in DMF.34 Satisfying result can also be achieved with the use of toluene (Table 1, Entry 2).19,20 1,4-Dioxane and water achieved high HMF conversion but low DFF selectivity (Table 1, Entries 4 and 5), which may be attributed to the decomposition of 1,4-dioxane molecule19 and the hydration of the aldehyde groups in HMF and DFF to germinal diols,21,59 respectively. Alcohol-type solvents (IPA and EtOH) also achieved great HMF conversion and selectivity toward DFF (Table 1, Entries 3 and 6).19 MeCN and DMSO attained modest level of HMF conversion (Table 1, Entries 7 and 8), the poor performance of DMSO may be ascribed to the strong coordination effect of DMSO molecule with Ru3+ center.20,60 Besides, it has been reported that DMSO tend to undergo disproportionation to yield toxic Me2SO2 and Me2S under oxidizing conditions.21
| Entry | Solvent | HMF conversion (%) | DFF yield (%) | DFF selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: HMF (63 mg, 0.5 mmol), Ru-PVP/CNT (60 mg, Ru 2.2 wt%), solvent (5 mL), O2 (2.0 MPa), 120 °C, 12 h. | ||||
| 1 | N,N-Dimethylformamide | >99 | 94 | 95 |
| 2 | Toluene | >99 | 87 | 88 |
| 3 | Isopropyl alcohol | >99 | 77 | 78 |
| 4 | 1,4-Dioxane | >99 | 35 | 35 |
| 5 | Water | >99 | 13 | 13 |
| 6 | Ethanol | 92 | 83 | 89 |
| 7 | Acetonitrile | 70 | 66 | 94 |
| 8 | Dimethylsulfoxide | 60 | 52 | 86 |
In addition to the solvent effect, the catalyst Ru-PVP/CNT loading level also exhibits a dramatic influence on the aerobic oxidation of HMF to DFF. As shown in Fig. 10, HMF conversions of 82% and 95% were obtained after 12 h with 40 mg and 50 mg of Ru-PVP/CNT, respectively, the corresponding DFF yields were 77% and 88%. Notably, HMF conversion and DFF yield increased to >99% and 94%, respectively, after 12 h by the use of 60 mg of Ru-PVP/CNT. The higher the catalyst loading, the higher both HMF conversion and DFF yield. The results indicate that the increase of the catalyst amount leads to the increase of the aerobic oxidation rate of HMF to DFF, which should be ascribed to the increase in the number and availability of catalytically active sites.34 Notably, the selectivity of DFF almost always maintained around 92–95% regardless of catalyst loading level (Fig. 10). This result suggests that the use of Ru-PVP/CNT can effectively avoid further oxidation of DFF to FFCA under the investigated conditions, thus leading to a high selectivity to DFF. In fact, the DFF selectivity is mainly determined by the reaction temperature, the solvent as well as the oxidant in this research.17,18,32
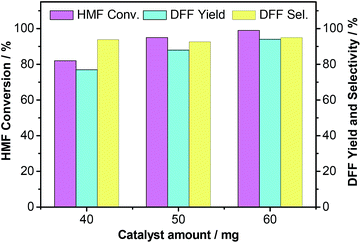 | ||
| Fig. 10 Aerobic oxidation of HMF to DFF with various catalyst dosages. Reaction conditions: HMF (63 mg, 0.5 mmol), Ru-PVP/CNT (Ru 2.2 wt%), DMF (5 mL), O2 (2.0 MPa), 120 °C, 12 h. | ||
In general, oxidant plays a vital role on the conversion rate of HMF and selectivity to DFF. As shown in Table 2, molecular oxygen, the clean and easily available oxidant, was most effective for the oxidation of HMF to DFF. An increase of the oxygen pressure leads to an increase of both HMF conversion and DFF yield (Table 2, Entries 1–4), which is ascribed to an enhanced molecular oxygen concentration in the solvent with the increase of oxygen pressure.20,34 In addition to O2, common oxidants such as tert-butyl hydroperoxide (t-BuOOH) and hydrogen peroxide (H2O2) were also explored for the oxidation of HMF to DFF. However, DFF yield was rather low with t-BuOOH as the oxidant though nearly quantitative HMF conversion was observed (Table 2, Entry 5), which can be attributed to the strong oxidative capability of t-BuOOH leading to the breakage of the furan ring.17,18 By contrast, low HMF conversion of 47% and low DFF yield of 32% were obtained by using H2O2 (Table 2, Entry 6), which is presumably attributed to the quick decomposition of H2O2 catalyzed by ruthenium species.17,61 Therefore, the mild oxidant H2O2 was not effective for the oxidation of HMF to DFF, which is consistent with the previous results.18,32
| Entry | Oxidant | HMF conversion (%) | DFF yield (%) | DFF selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: HMF (63 mg, 0.5 mmol), Ru-PVP/CNT (60 mg, Ru 2.2 wt%), DMF (5 mL), 120 °C, 12 h.b O2 (2.0 MPa).c O2 (1.0 MPa).d O2 (0.5 MPa).e O2 (0.1 MPa).f t-BuOOH (3.5 mmol).g H2O2 (3.5 mmol) were used, respectively. | ||||
| 1 | O2b | >99 | 94 | 95 |
| 2 | O2c | 95 | 90 | 95 |
| 3 | O2d | 83 | 80 | 96 |
| 4 | O2e | 26 | 25 | 96 |
| 5 | t-BuOOHf | 98 | 13 | 13 |
| 6 | H2O2g | 47 | 32 | 68 |
In addition to Ru-PVP/CNT, various other ruthenium catalysts, made of Ru3+ immobilized on PVP and PVI/CNT (Fig. 1), were further investigated to probe the influence of support on the selective aerobic oxidation of HMF to DFF (Fig. 11). Without immobilization of Ru3+, no oxidation products were detected with the use of pristine PVP/CNT in the blank experiment (Fig. 11), which indicated that the Ru rather than the support PVP/CNT showed catalytic activity in the aerobic oxidation of HMF.19 In the case of Ru-PVP and Ru-PVI/CNT, moderate HMF conversions and DFF yields were observed. Obviously, among all the catalysts explored, Ru-PVP/CNT is considerably more active and selective to afford DFF from HMF under the investigated conditions, presumably owing to the strong coordination ability of the pyridyl ligand to the ruthenium ion in the Ru-PVP/CNT. The decrease in HMF conversion with recycled Ru-PVP/CNT may be attributed to the leaching of active metal (ICP-OES analysis of the recycled Ru-PVP/CNT showed that 1.9 wt% of Ru was immobilized on PVP/CNT), the inevitable losses of catalyst during filtration18 and the insoluble polymeric furanic compounds adsorbed on the catalyst.19
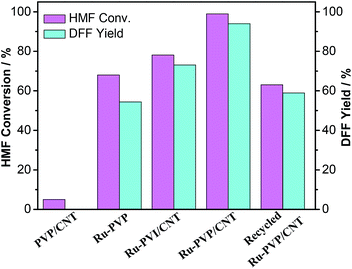 | ||
| Fig. 11 Aerobic oxidation of HMF to DFF with various catalysts. Reaction conditions: HMF (63 mg, 0.5 mmol), catalyst (60 mg), DMF (5 mL), O2 (2.0 MPa), 120 °C, 12 h. | ||
3. Conclusions
In conclusion, the composite material of poly(4-vinylpyridine)-functionalized carbon-nanotube (PVP/CNT) was prepared by in situ polymerization of 4-vinylpyridine monomer in the presence of CNT suspension. Raman spectra analysis confirmed the almost unchanged graphitized surfaces of CNT moiety in the PVP/CNT after the covalent functionalization of pristine CNT with PVP. The catalyst of ruthenium complex immobilized on PVP/CNT (Ru-PVP/CNT) shows excellent catalytic performance towards selective aerobic oxidation of biomass-based 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF). Under the optimal condition, a DFF yield of 94% with a full HMF conversion were obtained by using Ru-PVP/CNT in N,N-dimethylformamide (DMF) under 2.0 MPa O2 after 12 h at 120 °C. The research highlights a good prospect for catalytic application of polymer/CNT composite material for biomass-related transformations.4. Experiment
4.1 Materials and characterization
Materials and catalyst characterizations are provided in the ESI.†4.2 Catalyst preparation
4.3 Typical procedures for the aerobic oxidation of HMF to DFF
Aerobic oxidation of HMF was carried out in Teflon-lined stainless steel autoclave (50 mL). Typically, HMF (63 mg, 0.5 mmol) and Ru-PVP/CNT (60 mg, Ru 2.2 wt%) was added into DMF (5 mL), and the mixtures were stirred at ca. 700 rpm during the reactions. The reaction was carried out at a given reaction time at 120 °C under 2.0 MPa O2. After the reaction, the mixture was cooled to ambient temperature. The quantitative analysis methods of substrate and product are provided in the ESI.†4.4 Reusability of the catalyst
The resulting precipitates were filtrated from the reaction mixture by nylon membrane and washed with de-ionized water. The solid powder was then dried in a vacuum oven at 60 °C until it attained constant weight. The recovered catalyst was used for the next circle, and other steps were the same as those described above.Acknowledgements
We are grateful for the financial support from National Natural Science Foundation of China (21472189, 21172219, 51406211 and 21207039), National Basic Research Program of China (973 Program, 2012CB215304), 100 Talents Program of the Chinese Academy of Sciences, and Guangdong Natural Science Foundation (S2013010012986, S2011010000737 and S2013040012615).Notes and references
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS PubMed.
- R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- K. T. Hopkins, W. D. Wilson, B. C. Bender, D. R. McCurdy, J. E. Hall, R. R. Tidwell, A. Kumar, M. Bajic and D. W. Boykin, J. Med. Chem., 1998, 41, 3872–3878 CrossRef CAS PubMed.
- M. D. Poeta, W. A. Schell, C. C. Dykstra, S. Jones, R. R. Tidwell, A. Czarny, M. Bajic, A. Kumar, D. Boykin and J. R. Perfect, Antimicrob. Agents Chemother., 1998, 42, 2495–2502 Search PubMed.
- A. S. Amarasekara, D. Green and L. D. Williams, Eur. Polym. J., 2009, 45, 595–598 CrossRef CAS PubMed.
- D. T. Richter and T. D. Lash, Tetrahedron Lett., 1999, 40, 6735–6738 CrossRef CAS.
- A. S. Amarasekara, D. Green and E. McMillan, Catal. Commun., 2008, 9, 286–288 CrossRef CAS PubMed.
- T. El-Hajj, J. C. Martin and G. Descotes, J. Heterocycl. Chem., 1983, 20, 233–235 CrossRef CAS.
- J. W. Van Reijendam, G. J. Heeres and M. J. Janssen, Tetrahedron, 1970, 26, 1291–1301 CrossRef CAS.
- L. Cottier, G. Descotes, J. Lewkowski and R. Skowroñski, Org. Prep. Proced. Int., 1995, 27, 564–566 CrossRef CAS.
- L. Cottier, G. Descotes, E. Viollet, J. Lewkowski and R. Skowroñski, J. Heterocycl. Chem., 1995, 32, 927–930 CrossRef CAS.
- B. Karimi, H. M. Mirzaei and E. Farhangi, ChemCatChem, 2014, 6, 758–762 CrossRef CAS.
- N. Mittal, G. M. Nisola, L. B. Malihan, J. G. Seo, S. P. Lee and W. J. Chung, Korean J. Chem. Eng., 2014, 31, 1362–1367 CrossRef CAS PubMed.
- W. Partenheimer and V. V. Grushin, Adv. Synth. Catal., 2001, 343, 102–111 CrossRef CAS.
- Y. M. Wang, B. Liu, K. C. Huang and Z. H. Zhang, Ind. Eng. Chem. Res., 2014, 53, 1313–1319 CrossRef CAS.
- S. G. Wang, Z. H. Zhang, B. Liu and J. L. Li, Ind. Eng. Chem. Res., 2014, 53, 5820–5827 CrossRef CAS.
- C. A. Antonyraj, J. Jeong, B. Kim, S. Shin, S. Kim, K. Y. Lee and J. K. Cho, J. Ind. Eng. Chem., 2013, 19, 1056–1059 CrossRef CAS PubMed.
- J. F. Nie, J. H. Xie and H. C. Liu, J. Catal., 2013, 301, 83–91 CrossRef CAS PubMed.
- J. F. Nie, J. H. Xie and H. C. Liu, Chin. J. Catal., 2013, 34, 871–875 CrossRef CAS.
- I. Sádaba, Y. Y. Gorbanev, S. Kegnaes, S. S. R. Putluru, R. W. Berg and A. Riisager, ChemCatChem, 2013, 5, 284–293 CrossRef.
- N.-T. Le, P. Lakshmanan, K. Cho, Y. Han and H. Kim, Appl. Catal., A, 2013, 464–465, 305–312 CrossRef CAS PubMed.
- F. L. Grasset, B. Katryniok, S. Paul, V. Nardello-Rataj, M. Pera-Titus, J. M. Clacens, F. D. Campo and F. Dumeignil, RSC Adv., 2013, 3, 9942–9948 RSC.
- J. F. Nie and H. C. Liu, Pure Appl. Chem., 2012, 84, 765–777 CAS.
- J. P. Ma, Z. T. Du, J. Xu, Q. H. Chu and Y. Pang, ChemSusChem, 2011, 4, 51–54 CrossRef CAS PubMed.
- C. Carlini, P. Patrono, A. M. R. Galletti, G. Sbrana and V. Zima, Appl. Catal., A, 2005, 289, 197–204 CrossRef CAS PubMed.
- C. Moreau, R. Durand, C. Pourcheron and D. Tichit, Stud. Surf. Sci. Catal., 1997, 108, 399–406 CrossRef CAS.
- O. C. Navarro, A. C. Canós and S. I. Chornet, Top. Catal., 2009, 52, 304–314 CrossRef CAS.
- X. Q. Jia, J. P. Ma, M. Wang, Z. T. Du, F. Lu, F. Wang and J. Xu, Appl. Catal., A, 2014, 482, 231–236 CrossRef CAS PubMed.
- C. A. Antonyraj, B. Kim, Y. Kim, S. Shin, K.-Y. Lee, I. Kim and J. K. Cho, Catal. Commun., 2014, 57, 64–68 CrossRef CAS PubMed.
- B. Liu, Z. H. Zhang, K. L. Lv, K. J. Deng and H. M. Duan, Appl. Catal., A, 2014, 472, 64–71 CrossRef CAS PubMed.
- J. F. Nie and H. C. Liu, J. Catal., 2014, 316, 57–66 CrossRef CAS PubMed.
- G. D. Yadav and R. V. Sharma, Appl. Catal., B, 2014, 147, 293–301 CrossRef CAS PubMed.
- G. A. Halliday, R. J. Young and V. V. Grushin, Org. Lett., 2003, 5, 2003–2005 CrossRef CAS PubMed.
- X. Xiang, L. He, Y. Yang, B. Guo, D. M. Tong and C. W. Hu, Catal. Lett., 2011, 141, 735–741 CrossRef CAS.
- H.-J. Yoon, J.-W. Choi, H.-S. Jang, J.-W. Byun, W.-J. Chung, S.-M. Lee and Y.-S. Lee, Synlett, 2011, 165–168 CAS.
- A. Takagaki, M. Takahashi, S. Nishimura and K. Ebitani, ACS Catal., 2011, 1, 1562–1565 CrossRef CAS.
- R. L. Liu, J. Z. Chen, L. M. Chen, Y. Y. Guo and J. W. Zhong, ChemPlusChem, 2014, 79, 1448–1454 CrossRef CAS.
- J. Z. Chen, Y. Y. Guo, J. Y. Chen, L. Song and L. M. Chen, ChemCatChem, 2014, 6, 3174–3181 CrossRef CAS.
- N. Karousis and N. Tagmatarchis, Chem. Rev., 2010, 110, 5366–5397 CrossRef CAS PubMed.
- J. Z. Chen, W. Zhang, L. M. Chen, L. L. Ma, H. Gao and T. J. Wang, ChemPlusChem, 2013, 78, 142–148 CrossRef CAS.
- A. B. Chen, Y. L. Li, J. Z. Chen, G. Y. Zhao, L. L. Ma and Y. F. Yu, ChemPlusChem, 2013, 78, 1370–1378 CrossRef CAS.
- R. L. Liu, J. Z. Chen, X. Huang, L. M. Chen, L. L. Ma and X. J. Li, Green Chem., 2013, 15, 2895–2903 RSC.
- J. M. Clear, J. M. Kelly and J. G. Vos, Macromol. Chem. Phys., 1983, 184, 613–625 CrossRef CAS.
- N. H. Agnew, J. Polym. Sci., Polym. Chem. Ed., 1976, 14, 2819–2830 CrossRef CAS.
- L. A. Belfiore, M. P. McCurdie and E. Ueda, Macromolecules, 1993, 26, 6908–6917 CrossRef CAS.
- S. W. Kshirsagar, N. R. Patil and S. D. Samant, Synth. Commun., 2010, 40, 407–413 CrossRef CAS.
- P. Mondal, S. P. Bayen, K. Roy and P. Chowdhury, Sep. Sci. Technol., 2012, 47, 1651–1659 CrossRef CAS.
- M. M. Mdleleni, R. G. Rinker and P. C. Ford, Inorg. Chim. Acta, 1998, 270, 345–352 CrossRef CAS.
- A. J. Pardey, A. D. Rojas, J. E. Yánez, P. Betancourt, C. Scott, C. Chinea, C. Urbina, D. Moronta and C. Longo, Polyhedron, 2005, 24, 511–519 CrossRef CAS PubMed.
- S. H. Qin, D. Q. Qin, W. T. Ford, J. E. Herrera and D. E. Resasco, Macromolecules, 2004, 37, 9963–9967 CrossRef CAS.
- M. G. H. Zaidi, T. Agarwal, S. Alam and A. K. Rai, Fullerenes, Nanotubes, Carbon Nanostruct., 2011, 19, 329–342 CrossRef CAS.
- H. B. Friedrich and N. Singh, Catal. Lett., 2006, 110, 61–70 CrossRef CAS PubMed.
- G. P. Rachiero, U. B. Demirci and P. Miele, Catal. Today, 2011, 170, 85–92 CrossRef CAS PubMed.
- H. Y. H. Chan, C. C. Takoudis and M. J. Weaver, J. Catal., 1997, 172, 336–345 CrossRef CAS.
- G. P. Williams, CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, 2001, p. 200 Search PubMed.
- T. Komanoya, H. Kobayashi, K. Hara, W.-J. Chun and A. Fukuoka, Appl. Catal., A, 2011, 407, 188–194 CrossRef CAS PubMed.
- S. E. Davis, B. N. Zope and R. J. Davis, Green Chem., 2012, 14, 143–147 RSC.
- K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657–10666 CrossRef CAS PubMed.
- P. C. Selvaraj and V. Mahadevan, J. Mol. Catal. A: Chem., 1997, 120, 47–54 CrossRef CAS.
- M. Takafuji, S. Ide, H. Ihara and Z. H. Xu, Chem. Mater., 2004, 16, 1977–1983 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14592e |
| This journal is © The Royal Society of Chemistry 2015 |