Corrosion behavior of a bipolar plate of carbon–polythene composite in a vanadium redox flow battery
Abstract
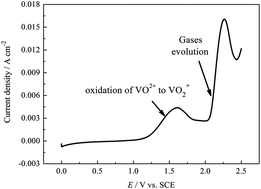
The corrosion behavior of the bipolar plate of carbon–polythene composite (BPCPC) and the effect of corrosion on the mechanical strength of BPCPC in a vanadium redox flow battery are investigated by electrochemical methods. The results show that when the anodic polarization potential is above 2.0 V, the BPCPC electrode can be eroded due to the gases' (including CO2, CO, and O2) evolution. The conductive network of BPCPC can also be destroyed, which includes the oxidation of the carbon atoms both in polythene and the carbon conductive additive to R–OH + C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH forms during corrosion. The damage of the conductive network leads to a decrease of mechanical strength and electroconductibility of BPCPC. Moreover, bulges and cracks are formed on the surface of BPCPC electrode exposure in the electrolyte, which may lead to battery failure because of electrolyte leakage.
C–OH forms during corrosion. The damage of the conductive network leads to a decrease of mechanical strength and electroconductibility of BPCPC. Moreover, bulges and cracks are formed on the surface of BPCPC electrode exposure in the electrolyte, which may lead to battery failure because of electrolyte leakage.

 Please wait while we load your content...
Please wait while we load your content...