Solubility properties and spectral characterization of sulfur dioxide in ethylene glycol derivatives†
Shaoyang Suna,
Yanxia Niuab,
Zuchen Suna,
Qiuxia Xuc and
Xionghui Wei*a
aDepartment of Applied Chemistry, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: xhwei@pku.edu.cn; Fax: +86-010-62670662; Tel: +86-010-62670662
bCollege of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China
cCollege of Chemical Engineering, Inner Mongolia University of Technology, Huhhot 010051, China
First published on 22nd December 2014
Abstract
Solubilities of SO2 in ethylene glycol derivatives were determined by dynamic isothermal gas–liquid equilibrium (GLE) experiments, and the thermodynamic parameters of the absorption processes were calculated. The GLE results indicated that the solubilities of SO2 in ethylene glycol derivatives increase in the order: diols < monomethyl ethers < dimethyl ethers, with the enthalpy values ranging from −23.2 to −43.3 kJ mol−1. The regeneration experiment found that the absorption of SO2 in tetraethylene glycol dimethyl ether is reversible, and the solvents can be reused without a significant loss of absorption capacity. The interactions between SO2 and ethylene glycol derivatives were investigated by UV, IR and NMR. In addition, a 1H-NMR spectroscopy technique with external references was used to investigate the physical absorption process of SO2 for the first time, in order to avoid the influence of deuterated solvents. Spectroscopic investigations showed that the interactions between SO2 and ethylene glycol derivatives are based on both the charge-transfer interaction and hydrogen bond. Ethylene glycol derivatives with desirable absorption capacities and excellent regeneration abilities are promising alternatives to conventional sorbents in SO2 separation.
1. Introduction
Sulfur dioxide (SO2), mainly emitted from the combustion of fossil fuels, has been one of the most important air pollutants, which causes serious damage to the environment and human health.1 Hence, the removal of SO2 from flue gas has become a global concern. The conventional technology widely used over the past decades is limestone scrubbing.2 However, it still has some drawbacks, including irreversible process, low efficiency and production of useless byproducts like waste water and CaSO4. Accordingly, new sorbents which can absorb SO2 efficiently, reversibly and selectively are still needed.Due to their excellent properties, such as negligible vapor pressure, wide liquid temperature range, high thermal stability and tunable structure, ionic liquids have been broadly studied in absorption of SO2.3,4 In 2004, 1,1,3,3-tetramethylguanidinium lactate [TMG][L] was first noted for SO2 removal, and the result showed that the ionic liquid can absorb about 1 mole SO2 per mole IL at 1 bar with 8% SO2 in gas phase.5 Later, numerous ILs based on guanidinium,6,7 alkanolaminium,8,9 imidazolium,10–12 pyridinium13 and phosphonium14 have been synthesized and applied in the SO2 removal. Recently, ether-functionalized15–21 and anion-functionalized task-specific ionic liquids22–24 were discovered to improve the SO2 absorption capacity, which is attributed to the multiple binding sites for SO2 in the functionalized molecules. Nevertheless, the industrial applications of ionic liquids have been limited by their high expenses and viscosities.
High-boiling solvents with low vapor pressures and proper viscosities are valuable solvents for flue gas desulfurization. In previous work, solubilities of SO2 in ethylene glycol and poly(ethylene glycol) have been determined, and the absorption mechanism was discussed.25–28 However, as far as we know, few comparisons of the absorption capacity and interaction mechanism have been made among ethylene glycol derivatives. Besides that, an efficient way to explore the interactions between SO2 and the solvents in the physical absorption process is still in demand.
In the work, solubilities of sulfur dioxide in ethylene glycol derivatives were determined by isothermal gas–liquid equilibrium experiment at the temperature ranging from 293.15 to 313.15 K, and a constant total pressure of 122.7 kPa. Thermodynamic parameters were calculated based on the GLE data to investigate the absorption processes. Desorption experiments were also conducted to study the regeneration property. In addition, UV and IR spectra of SO2 in ethylene glycol derivates were recorded to study the interaction between SO2 and solvents by comparing the spectral changes with the polarity of solvents. In conventional 1H-NMR experiments, deuterated reagents are mixed with samples as the internal references, so the chemical absorption processes of SO2 can be analyzed according to the chemical shifts of hydrogen atoms. However, the polar deuterated solvents (d6-DMSO, CDCl3, etc.) affect the physical absorption processes obviously because of their significant absorptions of acid gases. Here, we introduce a 1H-NMR spectroscopy method with external references, which is employed in the investigation of interaction between SO2 and physical sorbents for the first time.
2. Experimental section
2.1. Materials
Ethylene glycol derivatives, including ethylene glycol (EG), diethylene glycol (DEG), triethylene glycol (TEG), ethylene glycol monomethyl ether (EGME), diethylene glycol monomethyl ether (DEGME), ethylene glycol dimethyl ether (EGDME), diethylene glycol dimethyl ether (DEGDME), triethylene glycol dimethyl ether (TriEGDME), and tetraethylene glycol dimethyl ether (TetraEGDME), were selected in the study. TriEGDME and TetraEGDME were purchased from Tokyo Chemical Industry CO. Ltd. and Alfa Aesar, respectively, while the other reagents were from Sinopharm Chemical Reagent Co. Ltd. All reagents were obtained in the highest purity grade possible, and directly used as received without further purification. Chromatographic grade ethanol and distilled water were also used in this work. A certified standard gas SO2 in N2 (ΦSO2 = 8 × 10−3), supplied by Beijing Gas Center, Peking University (China), were employed to determine the GLE data of dilute SO2 in ethylene glycol derivatives.2.2. Solubility measurements
Solubility data of dilute SO2 in ethylene glycol derivatives were measured by isothermal gas–liquid equilibrium experiments at the temperature ranging from 293.15 K to 313.15 K and a constant total pressure of 122.7 kPa. The experimental instrument and process, including data process, were identical to the literature.29,30Measuring temperature was kept constant by a circulation water bath with ±0.01 K uncertainty. The system pressure was determined by a pressure gauge with an accuracy of ±0.1 kPa. The relative uncertainty of SO2 concentration in the liquid phase was estimated to be ±0.6%. The mass of samples were determined with an analytical balance (Sartorius BS 224S), and the uncertainty is ±0.0001 g.
2.3. Desorption experiment
Desorption experiment, which was conducted to investigate the regeneration property of tetraethylene glycol dimethyl ether, was carried out by bubbling N2 gas in flow rate of 200 mL min−1 through the sample absorbed SO2 (5 mL) at 343.15 K. The concentrations of SO2 in liquid phase before and after desorption were determined with the iodometric method.2.4. Spectral measurements
Spectrometric methods, including UV, IR and 1H-NMR, were used to investigate the mechanism of interactions between SO2 and ethylene glycol derivatives. UV-vis spectra were acquired with a UV-vis spectroscopy (UV 2401 PC Shimadzu). The IR spectra were recorded on a Bruker Vector 22 FT-IR spectrophotometer, in which a typical thin film method was performed at ambient condition, with the wavenumber ranging from 400 cm−1 to 4000 cm−1 and a resolution of 1 cm−1. A 500 MHz Bruker Avance III spectrometer was used to conduct the 1H-NMR experiments. The NMR experiments were performed with both of the internal and external references. For internal references, the sample was mixed in d6-DMSO or CDCl3. For external references, the samples and deuterated reagents were injected into capillary tubes (25 cm × 0.9 mm) and NMR tubes (17.8 cm × 5 mm), respectively. Then the capillary tube was inserted into the NMR tube to separate the samples from the solvents (deuterated regents).3. Results and discussion
3.1. Solubility data
A series of gas–liquid equilibrium (GLE) data of dilute SO2 in ethylene glycol derivatives were measured, and the results are listed in Table S1 (in ESI†). In the table, CSO2 and pSO2 denote the concentration of SO2 in liquid and the partial pressure of SO2 in gas phase, respectively.The GLE data of SO2 in ethylene glycol derivatives at 293.15 K are plotted in Fig. 1, with the partial pressure of SO2 in gas phase ranging from 0 to 130 Pa (figures of GLE data at other temperatures are shown from Fig. S1 to S4 in ESI†). It displays that the partial pressure of SO2 in gas phase is proportional to the concentration of SO2 in liquid within the range of investigated partial pressure. And the linear extrapolation curves pass through the zero point for all ethylene glycol derivatives, which demonstrates that the absorptions of SO2 in these solvents are typical physical processes and obey the Henry's law. In Fig. 1, it's obvious that the solubilities of SO2 in these solvents increase in the order: EG < DEG < TEG < DEGME < EGME < TetraEGDME < TriEGDME < DEGDME < EGDME. According to the results, we can divide these solvents into three categories: diols, monomethyl ethers and dimethyl ethers, and the solubility is improved via the substitution of hydroxyl group by methoxy group and the increasing numbers of ethylene glycol monomer (for diols and monomethyl ethers), which is consistent with the previous results of EG and PEG in literature.26,27 As a conclusion, ethers show better absorption abilities than alcohols.
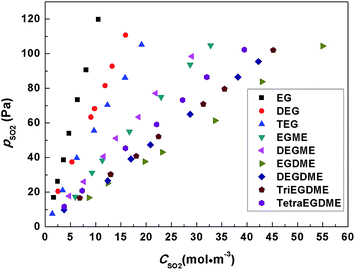 | ||
| Fig. 1 Solubility plots of dilute sulfur dioxide in ethylene glycol derivatives at 293.15 K and 122.7 kPa. | ||
Dimroth and Reichardt have proposed a parameter, ET(30), to estimate the solvent polarity based on the transition energy for the absorption band of Reichardt's dye.31 The ET(30) values of EG, DEG, TEG, EGME, TriEGDME, DEGDME and EGDME are 56.3, 53.8, 52.8, 52.0, 38.9, 38.6 and 38.2 kcal mol−1, respectively, which means the polarity decreases in the order. However, the solubilities of SO2 in ethylene glycol derivatives are opposite to the changing trend of polarity. As a consequence, dipole–dipole interaction is not the mean factor of the absorption process, and hydrogen-bond and charge-transfer interaction should be taken into consideration.
3.2. Thermodynamical model
GLE data of dilute SO2 in pure EGDME at different temperatures are plotted in Fig. 2 as an example to investigate the absorption property changing with the temperature, and the results are fitted linearly at each temperature. It indicated that the solubility of SO2 in EGDME decreases with the increasing temperatures, which means heating can be used as an efficient method for the regeneration of solvents. | ||
| Fig. 2 Solubility curves of dilute sulfur dioxide in EGDME at temperatures ranging from 293.15 K to 313.15 K and a constant pressure of 122.7 kPa. | ||
Considering that the absorptions of SO2 in ethylene glycol derivatives are typical physical processes as mentioned above, Henry's law constant (H′), Gibbs free energy (ΔG), enthalpy changes (ΔH) and entropy changes (ΔS) were calculated based on the GLE data with the data treatment method in literature30 (see Table S2 in ESI†). All thermodynamic parameters are listed in Table 1. It demonstrates that the absorptions are exothermic and enthalpy driving at investigated condition. The values of enthalpy are between −20 kJ mol−1 and −45 kJ mol−1, and increase in the order: diols < monomethyl ethers < dimethyl ethers, which are consistence with the solubility results. As in literature,28 the absorption process of SO2 in EG or PEG is based on both of the charge-transfer interaction and hydrogen bonding. The entire process can be divided into two steps: first, the absorption of SO2 induces the deposition of intermolecular hydrogen bond in pure EG or PEG; then SO2 molecules interact with EG or PEG molecules. Comparing the enthalpy of SO2 absorbed in diethers with in diols, it indicates that the existence of hydrogen bond (or hydroxyl group) is unfavorable for the absorption, mainly owing to the deposition energy of hydrogen bonds in the first step. *At the temperature of 293.15 K.
3.3. Desorption result
After the absorption of SO2, desorption of the high-boiling EG derivative, TetraEGDME, was conducted by heating and N2 bubbling. The desorption result shows that the 100% SO2 molecules can be regenerated in 30 min under the given condition, which means that the SO2 absorbed can be recycled, and the solvent can be reused. To evaluate the recyclability of the solvent, five cycles of absorption and desorption were conducted with the regenerated solvent without further purification (see Fig. S5 in ESI†). The result demonstrates that the solvent shows favorable recyclability, and is a promising alternative in industrial.3.4. UV spectroscopy analyses
Absorption spectra of SO2 absorbed in ethylene glycol derivatives were measured. For each spectrum, solvents with increasing concentrations of SO2 absorbed were detected, with the pure solvent as a reference. Since it's difficult to measure the SO2 absorption spectrum in gas phase, a spectrum of SO2 in n-C6H14 was measured instead, working as a reference to investigate the spectral changes caused by physical intermolecular interactions between SO2 and polar ethylene glycol derivatives.Absorptions of SO2 in n-C6H14 and typical ethylene glycol derivatives were shown in Fig. 3. Two characteristic absorption bands are observed, which belong to the electronic transition of π → π* for SO2 or n → σ* for oxygen atom in derivatives (shorter wavelength band) and n → π* for SO2 (longer wavelength band), and the intensities of both the bands increase with the concentration of SO2. The position of shorter wavelength band moves to long wavelength with the increasing concentrations of SO2, which is more significant when the ratio of hydroxyl group goes up in the derivative. It's attribute to the bathochromic shift of n → σ* transition with the formation of hydrogen bond between sulfur dioxide and hydroxyl group in diols.32,33
In contrast to the apolar solvents (n-C6H14), relative band intensity of n → π* absorption band to π → π* absorption band increases significantly as the polarity of solvent increases, which obeys the Ham effect.34 Meanwhile, the position of n → π* absorption band moves to a shorter wavelength in ethylene glycol derivatives, and the wavelengths of EG, EGME, EGDME, n-C6H14 are 275 nm, 276.5 nm, 277 nm and 288.5 nm, respectively, which is consistence with the ET(30) values measured by Reichardt's dye (56.3, 52.0, 38.2, and 31.0 kcal mol−1).35 For n → π* absorption band, the interaction between sulfur atoms in SO2 and oxygen atoms in derivatives promotes to the stabilization of n nonbonding orbital rather than π* antibonding orbital. In addition, hydroxyl group are capable of the hydrogen bond formation, lowing the energy of n orbital.36 Both of the effects cause the hypochromatic shift. As a result, charge-transfer interaction and hydrogen bonding exist in the solutions of SO2 in ethylene glycol derivatives.
3.5. IR analysis
IR spectra of SO2 in ethylene glycol derivatives were recorded to investigate the interactions, and typical spectra are shown in Fig. 4. As in literature,37 liquid SO2 has three fundamental vibrational frequencies: 1361.76 cm−1, 1151.38 cm−1 and 517.69 cm−1, which is attribute to asymmetrical stretching vibration (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) as), symmetrical stretching vibration(
as), symmetrical stretching vibration(![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) s) and bending vibration (γ), respectively.37 As shown in figures, the asymmetrical stretching vibrations and bending vibrations of SO2 in each solvent can be seen obviously, while symmetrical stretching vibrations in some solvents are covered by the stretching vibrations of C–O–C. According to the results, bending vibration wavenumbers of SO2 in ethylene glycol derivations increase with the solvent polarity increasing (from 527 cm−1 in ethers to 528.5 cm−1 in diols), in contrast, asymmetrical stretching vibration wavenumbers decreases (from 1327 cm−1 in ethers to 1323 cm−1 in diols), as a result of the charge-transfer interaction and hydrogen bond between SO2 and the derivatives, conforming to the vibration shift rule in polar solvents.38
s) and bending vibration (γ), respectively.37 As shown in figures, the asymmetrical stretching vibrations and bending vibrations of SO2 in each solvent can be seen obviously, while symmetrical stretching vibrations in some solvents are covered by the stretching vibrations of C–O–C. According to the results, bending vibration wavenumbers of SO2 in ethylene glycol derivations increase with the solvent polarity increasing (from 527 cm−1 in ethers to 528.5 cm−1 in diols), in contrast, asymmetrical stretching vibration wavenumbers decreases (from 1327 cm−1 in ethers to 1323 cm−1 in diols), as a result of the charge-transfer interaction and hydrogen bond between SO2 and the derivatives, conforming to the vibration shift rule in polar solvents.38
In addition, stretching vibration wavenumbers of C–O and O–H are constant in diols or monomethyl ethers before and after SO2 absorption, which demonstrates that the absorption process of SO2 absorbed in these solvents is the deformation of hydrogen bond in solvents, and the formation of intermolecular hydrogen bond between SO2 and alcohols. However, the stretching vibration of C–O in ethylene glycol dimethyl ethers moves to lower wavenumbers after SO2 absorption, which is in agreement with the mechanism of charge-transfer interaction between SO2 and ethers.
3.6. NMR analysis
NMR experiments were conducted to investigate the absorption mechanism of SO2 in ethylene glycol derivatives, with deuterate regents as both internal and external references. The 1H-NMR spectra of TriEGDME before and after SO2 absorption are shown in Fig. 5. Compared the internal reference method before and after SO2 absorption, no significant chemical shift is observed, indicating that the absorption is a physical process without the formation of new compounds. For pure TriEGDME, the chemical shifts of 1H-NMR signals move upfield with an external reference. Meanwhile, the chemical shift for each hydrogen atom moves to a noticeable lower field after SO2 absorption with an external reference. Buckingham39 have suggested that apart from the electronic distribution in the molecule, the chemical shift δ of H atoms is still influenced by several factors:| Δδ = δobsd − δ0 = δb + δa + δw + δe + δs |
 | ||
| Fig. 5 The 1H-NMR spectra of TriEGDME with internal and external references before and after SO2 absorption (d6-DMSO as references). | ||
According to the equation, the differences of pure TriEGDME chemical shifts between internal and external references methods are based on both of the shape of the sample and dipole–dipole interaction between TriEGDME and d6-DMSO. For TriEGDME with the external reference method before and after SO2 absorption, the charge-transfer interaction between SO2 and TriEGDME induces the chemical shift moving highfield, theoretically. However, SO2 molecule with a Π34 bond induces a significant downfield movement by the aromatic ring current effect, just like the aromatic solvent-induced shift (ASIS) of benzene, which is the mean factor of the results here.40
1H-NMR spectroscopy experiments of DEGDME with different concentrations of SO2 were also conducted with D2O as a reference, and the spectra were shown in Fig. 6. When the concentration of SO2 increases, the NMR signals of all hydrogen atoms shift downfield with respect to the position of pure DEGDME. What's more, the chemical shifts is proportional to the SO2 concentration as shown in Fig. 6, so 1H-NMR is a promising method to the determination of SO2 concentrations in solvents.
 | ||
| Fig. 6 The 1H-NMR spectra and chemical shifts changes of DEGDME with different concentrations of SO2 absorbed (D2O as an external reference). | ||
The charge-transfer interaction between n-butyl ether and SO2 was also studied by 1H-NMR spectroscopy with external references (Fig. 7). The SO2-induced chemical shift changes of all hydrogen atoms in n-butyl ether (NBE) were calculated, and it demonstrates that the changes of chemical shifts are consistent with the distance between the hydrogen atoms and the oxygen atom, which interacts with the sulfur atom in SO2. Above all, the effect of charge-transfer interaction between sulfur dioxide and ethylene glycol diethers has been proven, and the chemical shift changes of hydrogen atoms in carbon atomic chains can be used as an indicator to investigate the binding sites of SO2 in ethylene glycol derivatives.
 | ||
| Fig. 7 1H-NMR spectra of n-butyl ether before and after SO2 absorption (D2O as an external reference). | ||
According to the 1H-NMR spectra of DEGDME absorbed SO2, the chemical shift changes per unit SO2 concentration (g SO2 per g solvent) of three hydrogen atoms are 0.214, 0.248 and 0.276, as the positions from the end to the center. It demonstrates that the interaction between sulfur atom in SO2 and the oxygen atom in the center oxygen atom in DEGDME. Another illustrative example is the interaction between SO2 and DEG, and the spectra were shown in Fig. 8. The chemical shift changes (Δδ) of hydrogen atoms are 0.099 (hydroxyl), 0.188 and 0.183. It means sulfur atoms in SO2 prefer to interact with the central oxygen atom, which is consistent with DEGDME.
The applications of 13C-NMR with external references were also taken into consideration. Compared the chemical shifts of DEGDME before and after SO2 absorption, the positions have a slight shift to a higher field due to the decrease of electron-withdrawing ability for ether groups, caused by the charge-transfer interaction (see Fig. S6 in ESI†). Considering that the chemical shifts in 13C-NMR cover a larger range, the influence of ASIS in 13C-NMR is not a significant factor as in 1H-NMR.
4. Conclusions
Solubilities of SO2 in ethylene glycol derivatives were determined by a dynamic isothermal gas–liquid equilibrium (GLE) experiment at the temperatures ranging from 293.15 K to 313.15 K and a constant pressure of 122.7 kPa. Thermodynamic parameters of the absorption process were calculated based on the GLE data. The GLE results indicate that the absorption properties of ethylene glycol derivatives increase in the order: diol < monomethyl ether < dimethyl ether, with the enthalpy values of the absorption process ranging from −23.2 to −43.3 kJ mol−1. The regeneration experiments found that the absorption of SO2 is reversible, and the solvents can be reused without a significant loss of absorption capacity for at least 5 recycles.The interactions between SO2 and derivatives were investigated by spectroscopy experiments including UV, IR and NMR techniques. Particularly, a novel 1H-NMR method with external references was employed in this work. Spectroscopic investigation showed that the interaction between SO2 and ethylene glycol derivatives is based on charge-transfer interaction between sulfur atoms (SO2) and oxygen atoms (–O–), and the hydrogen bond formed between sulfur dioxide and hydroxyl. Thus, the ethylene glycol derivatives with desirable absorption capacities and excellent regeneration abilities are promising alternatives to conventional sorbents in SO2 separation. More importantly, the 1H-NMR spectroscopy with external references has a potential application in the investigation of physical interactions between acid gases and solvents.
Acknowledgements
This work is supported by Boyuan Hengsheng High-Technology Co., Ltd., Beijing, China.Notes and references
- J. D. Spengler, B. G. Ferris Jr, D. W. Dockery and F. E. Speizer, Environ. Sci. Technol., 1979, 13, 1276–1280 CrossRef CAS.
- V. Kaplan, E. Wachtel and I. Lubomirsky, RSC Adv., 2013, 3, 15842–15849 RSC.
- K. Huang, G. N. Wang, Y. Dai, Y. T. Wu, X. B. Hu and Z. B. Zhang, RSC Adv., 2013, 3, 16264–16269 RSC.
- S. D. Tian, Y. C. Hou, W. Z. Wu, S. H. Ren and C. Zhang, RSC Adv., 2013, 3, 3572–3577 RSC.
- W. Wu, B. Han, H. Gao, Z. Liu, T. Jiang and J. Huang, Angew. Chem., Int. Ed., 2004, 43, 2415–2417 CrossRef CAS PubMed.
- G. Yu and X. Chen, J. Phys. Chem. B, 2011, 115, 3466–3477 CrossRef CAS PubMed.
- Y. Wang, C. Wang, L. Zhang and H. Li, Phys. Chem. Chem. Phys., 2008, 10, 5976 RSC.
- L. Z. Zhai, Q. Zhong, C. He and J. Wang, J. Hazard. Mater., 2010, 177, 807–813 CrossRef CAS PubMed.
- K. Huang, J. F. Lu, Y. T. Wu, X. B. Hu and Z. B. Zhang, Chem. Eng. J., 2013, 215, 36–44 CrossRef PubMed.
- J. Huang, A. Riisager, P. Wasserscheid and R. Fehrmann, Chem. Commun., 2006, 4027 RSC.
- M. B. Shiflett and A. Yokozeki, Energy Fuels, 2010, 24, 1001–1008 CrossRef CAS.
- A. Yokozeki and M. B. Shiflett, Energy Fuels, 2009, 23, 4701–4708 CrossRef CAS.
- S. J. Zeng, H. S. Gao, X. C. Zhang, H. F. Dong, X. P. Zhang and S. J. Zhang, Chem. Eng. J., 2014, 251, 248–256 CrossRef CAS PubMed.
- G. F. Qu, J. Zhang, J. Y. Li and P. Ning, Sep. Sci. Technol., 2013, 48, 2876–2879 CrossRef CAS.
- S. Y. Hong, J. Im, J. Palgunadi, S. D. Lee, J. S. Lee, H. S. Kim, M. Cheong and K.-D. Jung, Energy Environ. Sci., 2011, 4, 1802 CAS.
- Z. Z. Yang, L. N. He, Q. W. Song, K. H. Chen, A. H. Liu and X. M. Liu, Phys. Chem. Chem. Phys., 2012, 14, 15832–15839 RSC.
- Z. Z. Yang, L. N. He, Y. N. Zhao and B. Yu, Environ. Sci. Technol., 2013, 47, 1598–1605 CAS.
- L. H. Zhang, Z. J. Zhang, Y. L. Sun, B. Jiang, X. G. Li, X. H. Ge and J. T. Wang, Ind. Eng. Chem. Res., 2013, 52, 16335–16340 CrossRef CAS.
- Y. Zhao, J. Y. Wang, H. C. Jiang and Y. Q. Hu, J. Mol. Liq., 2014, 196, 314–318 CrossRef CAS PubMed.
- G. Cui, C. Wang, J. Zheng, Y. Guo, X. Luo and H. Li, Chem. Commun., 2012, 48, 2633–2635 RSC.
- S. K. Tang, G. A. Baker and H. Zhao, Chem. Soc. Rev., 2012, 41, 4030–4066 RSC.
- C. Wang, G. Cui, X. Luo, Y. Xu, H. Li and S. Dai, J. Am. Chem. Soc., 2011, 133, 11916–11919 CrossRef CAS PubMed.
- G. K. Cui, J. J. Zheng, X. Y. Luo, W. J. Lin, F. Ding, H. R. Li and C. M. Wang, Angew. Chem., Int. Ed., 2013, 52, 10620–10624 CrossRef CAS PubMed.
- C. M. Wang, J. J. Zheng, G. K. Cui, X. Y. Luo, Y. Guo and H. R. Li, Chem. Commun., 2013, 49, 1166–1168 RSC.
- Q. Li, J. B. Zhang, L. H. Li, Z. Q. He, X. X. Yang, Y. F. Zhang, Z. H. Guo and Q. C. Zhang, J. Phys. Chem. B, 2013, 117, 5633–5646 CrossRef CAS PubMed.
- J. B. Zhang, L. H. Liu, T. R. Huo, Z. Y. Liu, T. Zhang and X. H. Wei, J. Chem. Thermodyn., 2011, 43, 1463–1467 CrossRef CAS PubMed.
- J. B. Zhang, P. Y. Zhang, F. Han, G. H. Chen, L. W. Zhang and X. H. Wei, Ind. Eng. Chem. Res., 2009, 48, 1287–1291 CrossRef CAS.
- J. B. Zhang, Q. A. Li, Z. H. Guo, K. X. Li, M. D. Xu, N. Zhang, T. Zhang and X. H. Wei, Ind. Eng. Chem. Res., 2011, 50, 674–679 CrossRef CAS.
- Y. Niu, F. Gao, S. Sun, J. Xiao and X. Wei, Fluid Phase Equilib., 2013, 344, 65–70 CrossRef CAS PubMed.
- G. Lan, J. Zhang, S. Sun, Q. Xu, J. Xiao and X. Wei, Fluid Phase Equilib., 2014, 373, 89–99 CrossRef CAS PubMed.
- C. Reichardt, Chem. Soc. Rev., 1992, 21, 147–153 RSC.
- D. Stevenson, J. Am. Chem. Soc., 1962, 84, 2849–2853 CrossRef CAS.
- D. Stevenson, G. Coppinger and J. Forbes, J. Am. Chem. Soc., 1961, 83, 4350–4352 CrossRef CAS.
- J. S. Ham, J. Chem. Phys., 1953, 21, 756–758 CrossRef CAS PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- G. J. Brealey and M. Kasha, J. Am. Chem. Soc., 1955, 77, 4462–4468 CrossRef CAS.
- R. H. Maybury, S. Gordon and J. J. Katz, J. Chem. Phys., 1955, 23, 1277–1281 CrossRef CAS PubMed.
- D. K. Cha, A. A. Kloss, A. C. Tikanen and W. R. Fawcett, Phys. Chem. Chem. Phys., 1999, 1, 4785–4790 RSC.
- A. Buckingham, T. Schaefer and W. Schneider, J. Chem. Phys., 1960, 32, 1227–1233 CrossRef CAS PubMed.
- H. Stamm and H. Jackel, J. Am. Chem. Soc., 1989, 111, 6544–6550 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13874k |
| This journal is © The Royal Society of Chemistry 2015 |



