Design and fabrication of carbon fiber/carbonyl iron core–shell structure composites as high-performance microwave absorbers
Abstract
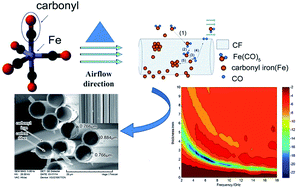
Microwave absorbing composites with carbon fiber (CF) and carbonyl iron (CI) particles as absorbers were prepared by a metal organic chemical vapor deposition (MOCVD) process. The structure and morphology analyses demonstrate that the composites have a complete core–shell structure with CF as core and CI layers as shell. The electromagnetic parameters can be adjusted by changing the CI content in the composites. Compared with CF, the composites have higher complex permeability and permittivity, which gradually increase with increasing CI content. When CF–CI = 1 : 8.8 (labeled as S2), the reflection loss (RL) of S2 exceeds −10 dB from 2 to 18 GHz for the absorber thickness between 0.9 and 3.9 mm, and a minimum RL value of −21.5 dB was observed at 6.6 GHz corresponding to a matching thickness of 2.0 mm. CI-doped CF by MOCVD can significantly improve the electromagnetic properties of CF and CF–CI composites could be used as an effective microwave absorption material.

 Please wait while we load your content...
Please wait while we load your content...