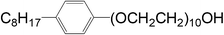Greatly enhanced porosity of stretched polypropylene/graphene oxide composite membrane achieved by adding pore-forming agent
Jian Daia,
Xian-ling Xua,
Jing-hui Yanga,
Nan Zhanga,
Ting Huanga,
Yong Wang*a,
Zuo-wan Zhoua and
Chao-liang Zhangb
aKey Laboratory of Advanced Technologies of Materials (Ministry of Education), School of Materials Science & Engineering, Southwest Jiaotong University, Chengdu, 610031, China. E-mail: yongwang1976@163.com; Tel: +86 28 87602714
bState Key Laboratory of Oral Diseases, Sichuan University, Chengdu, 610041, China
First published on 5th February 2015
Abstract
A small amount of graphene oxide (GO) was incorporated into polypropylene (PP) to prepare a composite membrane, assisted by the pore-forming agent polyoxyethyleneoctylphenyl-10 (OP-10). The composite membrane was obtained through melt-compounding and a subsequent tensile process. The dispersion of GO in the composite, the dynamic mechanical properties and the melting and crystallization behaviors of the melt-compounded samples were investigated to clearly understand the initial microstructures of the samples. Different tensile strains were applied to obtain the stretched PP composite membrane, and then the morphologies of the composite membrane and the porosity were comparatively investigated. The results showed that the dispersion of GO was apparently improved with the aid of OP-10 and many initial pores were simultaneously introduced into the PP/GO/OP-10 composite, which induced a slight decrease in the storage modulus and glass transition temperature of the PP matrix. OP-10 suppressed the crystallization of the PP matrix, while GO compensated for this effect. Both the stretched PP/OP-10 and the composite membranes exhibited larger mean pore sizes compared with the stretched pure PP membrane. Furthermore, compared with the stretched PP, PP/GO and PP/OP-10 membranes, greatly increased porosity was achieved for the stretched PP/GO/OP-10 composite membrane, especially at relatively high tensile strain. In addition, it was suggested that the initial pores, which were introduced by adding OP-10, acted as a stress concentrator, promoting the formation of more pores during the tensile process by inducing lamellar separation and breakage. This work provided a new method for the preparation of PP-based composite membranes and also endowed them with great potential in many fields.
1. Introduction
PP is regarded as an ideal raw material for the preparation of membranes due to its good processability, good mechanical properties, excellent chemical stability and thermal stability. To date, PP has been widely applied to porous membrane preparation mainly through thermally-induced phase separation1,2 and the stretching method.3–6 In terms of thermally-induced phase separation, PP and other components are first dissolved into a certain solvent to form a homogenous solution at high temperature, and the phase separation happens as the temperature decreases. Once the residual solvent is removed, the porous membrane is obtained.1,2 For the stretching method, the melting PP-based composite is treated to obtain a precursor membrane with an oriented structure, and then the precursor membrane is stretched at low or high temperature to induce the formation of pores through the breakage or separation of lamella.7,8 With the advantages of a simple preparation process, low cost, avoidance of solvent contamination and recovery and so on, the stretching method has attracted much more attention recently. The pore formation behavior of a PP membrane is greatly influenced by many factors, including material parameters3,9 and processing parameters.10–13As a new kind of functional nanofiller, graphene oxide (GO) exhibits a good reinforcement effect due to its excellent mechanical properties, and therefore, it has been widely used to prepare reinforced composites.14 GO also exhibits good absorbability, which endows it with great potential for application in waste water treatment.15–18 To date, introducing GO into membranes has attracted much attention from researchers, and many GO-based composite membranes have been developed, including poly(vinylidene fluoride)/GO,19–21 polyethersulfone/GO,22 and chitosan/graphene oxide.23 However, little work has been carried out to prepare a PP/GO composite membrane. S. Ramasundaram et al.24 prepared a PP fiber/reduced graphene oxide (rGO) composite membrane. rGO with a high coating density was well dispersed in the matrix through a three-step dip-coating method. The results showed that a highly hydrophobic surface was obtained and an excellent performance in terms of the water flux and trans-filter pressure was further demonstrated for the rGO-coated PP fiber membrane. However, it should be noted that the high hydrophobicity might lead to the contamination of the membrane surface. In addition, the complex preparation steps can not be suitable for membrane preparation. In our previous work,25 we attempted to prepare a β-nucleated PP/GO composite membrane using the stretching method. The results showed that the presence of GO resulted in the formation of pores with relatively smaller mean pore size under all conditions, especially at a relatively high stretching temperature and/or tensile speed, and it induced porosity enhancement in the stretched sample. However, it should be stressed that the porosity was not high enough, in spite of the presence of amounts of β-phase with a loose lamellae arrangement which usually facilitates the pore formation of a stretched membrane.10,26 Therefore, more work needs to be done to further enhance the porosity of the PP/GO composite membrane.
To obtain PP membranes with well-controlled structures and excellent comprehensive properties, lots of attempts have been made through compounding or blending with other components.27–32 For example, Villaluenga et al.27 prepared a PP/ethylene vinyl acetate copolymer (EVA)/clay composite membrane with the aid of a compatibilizer. Different morphologies were obtained by adjusting the content of the component and/or fillers, which resulted in a change in the selective gas permeability behavior. S. H. Tabatabaei et al.28 prepared a blend membrane using two linear PPs with different molecular weights through the stretching method. Bigger pore density and more uniform pore size were obtained as the amount of the high molecular weight component increased. Meanwhile, the interconnectivity of the pores also increased, which led to the improvement of permeability. What’s more, PP/diluent composite membranes were also widely prepared through the thermal phase separation method. Dibutyl phthalate (DBP),33 diamyl phthalate (DAP)34 and some other compounds were used as diluents in order to introduce pores during the phase separation process,35,36 and the pore formation behavior could be well controlled by adjusting the diluent content and thermal phase separation conditions.
Pore-forming agents, as effective additives for facilitating the pore formation of materials, are widely applied during the preparation and/or modification of porous membranes.37–39 With the assistance of a pore-forming agent, the structure of a matrix becomes loose, which is in favor of pore formation during the stretching process. Also, the stress field in the sample can be changed by introducing initial pores, which may trigger the formation of new pores around the initial pores during the stretching process.
In this work, we attempt to introduce a pore-forming agent, i.e. polyoxyethyleneoctylphenyl-10 (OP-10), into a PP/GO composite membrane. OP-10 is a type of amphiphilic pore-forming agent with methyl on one side and hydroxyl on the other. The pore formation mechanism is possibly related to the following two aspects.40 First, during the melt-compounding process, OP-10 has a tendency to distribute at the interface between the melt of PP and the air when the droplets of PP are melted. Under this condition, the migration of air becomes more difficult and it forms bubbles in the PP melt. Second, OP-10 also improves the stabilization of the bubbles. The effects of GO and OP-10 on the pore formation behaviors of PP, including the pore morphology and porosity obtained at different tensile strains, are investigated in detail. Interestingly, with the assistance of OP-10, greatly enhanced porosity is achieved for the stretched PP/GO/OP-10 composite membrane. This endows the PP/GO/OP-10 composite membrane with great potential for applications in many fields.
2. Experimental part
2.1 Materials
PP (trade name of PP140) with a melt flow rate (MFR) of 4.6 g per 10 min (190 °C, 2.16 kg) was obtained from Kaikai Petrochemical Corporation, China. Graphite was obtained from Qingdao Heilong Graphite Co., Ltd. The pore-foaming agent OP-10 was supplied by Chengdu Kelong Chemical Reagent Factory, China. The chemical structural formula of OP-10 is:2.2 Sample preparation
GO was prepared in our lab according to the modified Hummer’s method.41 After that, some functional groups, including carboxyl and hydroxyl groups, were introduced to the surface of the GO. The corresponding data relating to the microstructure of the GO can be seen in our previous work.42In this work, four different samples, i.e. neat PP, PP/GO containing 0.5 wt% GO, PP/OP-10 containing 5 wt% OP-10, and PP/GO/OP-10 containing 5 wt% OP-10 and 0.5 wt% GO were simultaneously prepared through the melt compounding process, which was conducted on a twin-screw extruder SHJ-20 (Nanjing Ruiya, China) at a screw speed of 200 rpm and with melt temperatures of 150–160–175–190–200–200–195 °C from hopper to die. In terms of the PP/GO-OP-10 sample, it is worth noting that before melt compounding of the sample, OP-10 and GO were first dissolved in Dimethyl Formamide (DMF) to improve the dispersion of GO. Then, the GO/OP-10 solution was poured into PP powder and the mixture was dried at 80 °C until the DMF was completely removed. After being granulated, the pellets of material were compression-molded at a melt temperature of 200 °C and a pressure of 5 MPa to obtain a dumbbell-shaped sample with a length of 112 mm, a width of 10 mm and a thickness of 0.1 mm.
2.3 Optical microscopy (OM)
Transmission optical microscopy (TOM) with an AXIO Imager A1m (ZERSS, Germany) was used to characterize the dispersion of GO in the samples. The sample slice was compression-molded using the extrudate and the thickness of the sample slice was about 20 μm.2.4 Transmission electron microscopy (TEM)
The dispersion of GO and the presence of initial pores in the composite were further investigated using transmission electron microscopy (TEM) with a JEM-2100F microscope (JEOL, Japan), with an operating voltage of 200 kV. An ultrathin section with a thickness of about 90 nm, which was cut using a cryo-diamond knife on a EM UC6/FC6 microtome (LEICA, Germany), was used for TEM characterization.2.5 Dynamic mechanical analysis (DMA)
The dynamic mechanical properties were measured by dynamic mechanical analysis (DMA) with a Q800 analyser (TA Instruments, USA). The tensile mode was selected. A rectangular sample, which was directly cut from the compression-molded bar, was used, with a length of 33 mm, a width of 10 mm and a thickness of about 0.1 mm. The measurements were carried out from −50 to 150 °C at a heating rate of 3 °C min−1 and a frequency of 1 Hz.2.6 Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) with a STA449C Jupiter (Netzsch, Germany) was used to investigate the crystalline structure of the sample. The sample without tensile measurement was heated from 30 to 200 °C at a heating rate of 10 °C min−1 and was maintained at 200 °C for 5 min to erase the thermal history, and then the sample was cooled down to 30 °C at a cooling rate of 5 °C min−1. The stretched sample was directly heated from 30 to 200 °C at the heating rate of 10 °C min−1. For each measurement, the weight of the sample was maintained at 8 mg and the measurements were carried out in a nitrogen atmosphere. The degree of crystallinity (Xc-DSC) can be calculated according to the following relation:
 | (1) |
2.7 Wide angle X-ray diffraction
The crystalline structures of the PP matrix in different samples were investigated using wide angle X-ray diffraction (WAXD, Panalytical X’pert PRO diffractometer with Ni-filtered Cu Kα radiation, the Netherlands). The continuous scanning angle range was set from 5° to 35° and the measurements were carried out at 40 kV and 40 mA. The degree of crystallinity (Xc-WAXD) was calculated according to the following relation:
 | (2) |
2.8 Tensile experiment
The tensile experiment was conducted on a M-4020 high-temperature creeping instrument (REGER, China). Different tensile strains (0%, 50%, 100% and 200%) were selected at the ambient temperature of 100 °C. After stretching, all the samples were maintained at 100 °C for 5 min to stabilize the pore morphology.2.9 Scanning electron microscopy (SEM)
The pore morphology was characterized using scanning electron microscopy (SEM) with a Fei Inspect microscope (FEI, the Netherlands) with an accelerating voltage of 20.0 kV. Before SEM characterization, all the samples were sputter-coated with a thin layer of gold. To accurately make a comparison of pore morphology among different samples, the mean pore size was calculated. It is worth noting that in the present work, the size of the pore was defined as the pore length along the tensile direction. When calculating the mean pore size, at least 200 pores from different zones of the sample were measured and the data were obtained using the Nano Measurer 1.2 software.2.10 Porosity measurement
The porosity (Ak) of the stretched membrane was measured through the following procedure: the stretched membrane was immersed in ethanol for 24 h; after that, the membrane was taken out and the ethanol on the membrane surface was carefully removed using filter paper. Finally, the treated membrane was weighed carefully. The porosity was calculated according to the following relation:44
 | (3) |
![[small rho, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d1.gif) (0.91 g cm−3) are the density of ethanol and PP, respectively.
(0.91 g cm−3) are the density of ethanol and PP, respectively.
3. Results and discussion
3.1 Microstructures of as prepared samples
The dispersion of GO in the PP/GO and PP/GO/OP-10 composites was firstly characterized using TOM. As shown in Fig. 1, GO exhibits relatively poor dispersion in the PP/GO composite. However, with the presence of OP-10, the dispersion of GO is apparently improved and the size of GO agglomerates is greatly decreased. To further understand the dispersion state of GO and the morphology of the initial pores in the sample, the representative PP/GO/OP-10 composite was further characterized using TEM. As shown in Fig. 2, at relatively low magnification, there are many initial pores in the sample, indicating that OP-10 is an effective pore-forming agent and that it successfully induces the formation of initial pores. Furthermore, one can see that the dispersion of initial pores is not homogeneous. Besides the presence of large pores with diameters up to 500 nm, some very small pores with a diameter of about 60 nm can be also seen. The sheet-like structure of GO can be differentiated at relatively high magnification. It is worth noting that GO is mainly dispersed around the initial pores. This is possibly related to the good interfacial interaction between OP-10 and GO. As shown previously, OP-10 is an amphiphilic pore-forming agent with methyl on one side and hydroxyl on the other. GO is also an amphiphilic filler with many functional groups on the edge of the sheet, including carboxyl and hydroxyl groups.45 Specifically, the sample preparation method that is carried out in the present work also provides a greater probability for the interaction between OP-10 and GO.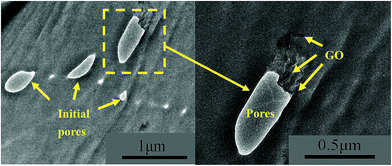 | ||
| Fig. 2 TEM images showing the dispersion of GO and the morphologies of the initial pores in the PP/GO/OP-10 composite. | ||
Fig. 3 shows the dynamic mechanical properties of all the samples. From Fig. 3a one can see that pure PP exhibits a glass transition temperature (Tg) of 12.8 °C. A slightly decreased Tg(12.0 °C) is observed for the PP/GO sample, possibly due to the slightly increased free volume induced by the presence of GO. A more apparent decrease in Tg is observed for the PP/OP-10 sample, which shows a Tg of 10.3 °C. This indicates that the chain segment mobility is enhanced due to the presence of OP-10. In other words, OP-10 can be thought of as a plasticizer of PP, possibly due to the presence of many pores that increase the free volume of the material, which is favorable for the motion of PP chain segments. The Tg of the PP/GO/OP-10 sample is slightly higher than that of the PP/OP-10 sample but is still smaller than those of pure PP and PP/GO samples. This is possibly related to the improved dispersion of GO particles that decrease the chain segment mobility of the PP matrix to a certain extent. It should be stressed that the measurement was repeated several times and a similar variation trend was observed. Therefore, the data have statistical significance. From Fig. 3b one can see that the presence of GO induces a slight decrease in the storage modulus (E′) of the PP/GO sample, but a more apparent decrease of E′ is observed for the PP/OP-10 sample, especially at temperatures below the glass transition temperature (Tg) of the PP matrix. This further indicates the plasticizing effect of OP-10. Over the whole measured temperature range applied in this work, the PP/GO/OP-10 composite exhibits a similar E′ to that of the PP/OP-10 sample. Generally, well-dispersed GO can exhibit a good reinforcement effect for a polymer.46 However, in this work, both for the PP/GO sample and for the PP/GO/OP-10 sample, the E′ is lower than that of the pure PP. This is possibly related to the relatively poor dispersion of GO in the PP/GO sample and the apparent plasticizing effect of OP-10 in the PP/GO/OP-10 sample.
 | ||
| Fig. 3 (a) Storage modulus and (b) mechanical loss factor of different samples as indicated in the graphs. | ||
The melting and crystallization behaviors of the different samples were investigated using DSC, and the results are shown in Fig. 4. From Fig. 4a one can see that pure PP exhibits a strong endothermic peak at 166.0 °C and a very weak peak at about 146.1 °C, attributed to the fusion of α-form PP (Tmα) and β-form PP (Tmβ), respectively. In terms of the PP/GO sample, double endothermic peaks are present at about 159.3 and 165.8 °C. This is consistent with the results reported in the literature that GO is an α-form nucleating agent of PP.47 For the PP/OP-10 sample, the intensity of the endothermic peak of β-form PP is increased, which indicates an increased amount of β-form PP in the sample. Furthermore, the endothermic peak of the α-form shifts to a lower temperature (161.4 °C), indicating that the lamellar thickness of α-form PP in the PP/OP-10 sample is smaller than that in the pure PP. Interestingly, compared with the PP/OP-10 sample, the PP/GO/OP-10 sample exhibits a weaker endothermic peak of β-form PP, on one hand. On the other hand, double endothermic peaks are present again at 161.1 and 165.0 °C. This further indicates that GO is an α-nucleating agent of PP and that it suppresses the nucleation and growth of β-form PP in the PP/GO/OP-10 sample. In other words, there is a competition between OP-10 and GO in inducing crystallization of the PP matrix. The crystallinity (Xc-DSC) was calculated and the results are also shown in Fig. 4a. One can see that pure PP exhibits the biggest Xc-DSC while the other three samples exhibit lower Xc-DSC values. This is also one of the reasons why the other three samples exhibit a smaller storage modulus compared with pure PP, as indicated in Fig. 3b.
As shown in Fig. 4b, compared with the crystallization of pure PP that exhibits a crystallization temperature (Tc) of 119.8 °C, the PP/GO sample exhibits a higher Tc (122.6 °C), further indicating the nucleation effect of GO on PP crystallization. However, the PP/OP-10 sample exhibits a lower Tc (117.6 °C). This implies that OP-10 suppresses the crystallization of the PP matrix. Because the crystallization of PP in the PP/OP-10 sample occurs at a relatively low temperature, the lamellar growth becomes more difficult. This is also the reason why the PP/OP-10 sample exhibits a lower Tmα compared with pure PP. Due to the nucleating effect of GO, the PP/GO/OP-10 sample exhibits a slightly enhanced Tc (120.6 °C).
To further understand the crystalline structure of the PP matrix in different samples, WAXD measurements were carried out. Fig. 5 shows the WAXD profiles of the different samples. It can be seen that pure PP exhibits several characteristic diffraction peaks at 2θ = 14.3°, 17.0°, 18.7°, 21.2° and 22.0°, attributed to the reflections of the (110), (040), (130), (111) and (131) crystal planes of α-form PP. In addition, a weak diffraction peak at 2θ = 16.0°, which is attributed to the reflection of the (300) crystal plane of β-form PP, is also observed, indicating the presence of a small amount of β-form PP in the pure PP. The PP/GO sample also exhibits the characteristic diffraction peaks of the α-form. Compared with pure PP, the PP/OP-10 sample exhibits a stronger diffraction peak of the (300) crystal plane, indicating an increased amount of β-form PP in the sample. This agrees well with the observation obtained from DSC measurements, as shown in Fig. 4a. The crystallinity of the sample was also calculated. As shown in Fig. 5, the four samples exhibit similar crystallinity. This is slightly different from the observations obtained through DSC measurements. The different variation trends of crystallinity between DSC measurements and WAXD measurements are possibly related to the different characterization methods.
 | ||
| Fig. 5 WAXD profiles showing the crystalline structure of different samples as indicated in the graph. | ||
3.2 Effect of OP-10 and GO on pore formation
The pore morphologies of the different samples obtained through the uniaxial tensile process were characterized using SEM. In this section, the tensile speed was set at 50 mm min−1, the ambient temperature was maintained at 100 °C, and the tensile strain was maintained at 100%. The representative SEM images are shown in Fig. 6 and the values of mean pore size are also labeled in the images. It can be clearly seen that many pores are successfully created in the stretched membranes. The pure PP membrane exhibits the smallest mean pore size of 193.8 nm, while the PP/GO and PP/OP-10 membranes exhibit bigger mean pore sizes of 223.3 and 260.5 nm, respectively. For the PP/GO/OP-10 composite membrane, the mean pore size is about 210.5 nm, which is slightly larger than that of the pure PP membrane but apparently smaller than that of the PP/OP-10 membrane. It is well known to all that the pore formation during the uniaxial stretching process is mainly attributed to the separation of lamellae along the tensile direction, which is also accompanied by deformation, rotation or breakage of a small amount of lamellae arranged along other directions.48 Furthermore, the molecular chain mobility also influences the nucleation and growth of the pores. Generally, during the tensile process, the enhanced mobility promotes the motion of molecular chains in the amorphous interlamellar region and the slippage and separation of the lamellar structure along the tensile direction, and then promotes the nucleation and growth of the pores. Therefore, the greatly increased mean pore size in the stretched PP/OP-10 membrane is mainly attributed to the enhanced chain mobility of the PP matrix, which has been shown by the decrease in Tg as shown in Fig. 3. Furthermore, the presence of β-form PP that exhibits a parallel lamellar stacking structure and the α-form with more defects and/or smaller lamellar thickness are possibly the other reasons for the greatly increased mean pore size. It has been demonstrated that pores are easily created through the slippage and separation of the parallel lamellar structure when the β-form PP is stretched.26,48–50 The PP/GO composite membrane exhibits a larger mean pore size than that of pure PP. This is possibly related to the presence of larger GO agglomerates, which result in a local stress concentration in the sample, facilitating the nucleation and growth of pores around the GO agglomerates through interfacial debonding between the PP matrix and GO agglomerates. For the PP/GO/OP-10 composite membrane, the presence of GO possibly restricts the motion of PP chain segments on one hand; on the other hand, GO suppresses the formation of β-form PP and promotes the integration of the α-form lamellar structure (seen in Fig. 4). Specifically, the dispersion of GO is apparently improved. Under this condition, the growth of pores becomes more difficult. However, the role of OP-10 can not be completely ignored. That is the reason why the mean pore size of the stretched PP/GO/OP-10 composite membrane is smaller than that of the PP/OP-10 membrane but is still larger than that of the pure PP membrane.The evolution of pore morphology during the tensile process of the representative PP/GO/OP-10 composite membrane was then characterized to determine the effect of tensile strain on the formation of pores. Fig. 7 shows the SEM images of the stretched membranes obtained at different tensile strains. The mean pore size of each sample is also shown in the corresponding image. From Fig. 7a one can see that before stretching, sporadic pores are present in the sample, which further demonstrates the pore-forming role of OP-10. After being stretched to a small strain, i.e. 50% (Fig. 7b), the stretched composite membrane exhibits at least two features. First, the initial pores are elongated along the tensile direction. Second, many small pores are introduced into the membrane. The average size of the new pores is about 118.8 nm. With an increase in tensile strain (100%, Fig. 7c), besides the further deformation of the initial pores during the tensile process, many more new pores are introduced on one hand. On the other hand, the mean pore size is increased up to 210.5 nm. This indicates that increasing the tensile strain facilitates the increase of pore size and pore number. However, it is worth noting that the increase in mean pore size becomes inconspicuous at a relatively high tensile strain. For example, at a tensile strain of 200% (Fig. 7d), the mean pore size is only increased up to 261.2 nm, which is only about 50 nm larger than that of the membrane obtained at a tensile strain of 100%.
The variation of porosity versus the tensile strain is illustrated in Fig. 8. For the stretched PP membrane, there is very little variation in porosity. Specifically, it can be seen that a relatively high tensile strain even induces a slight decrease in porosity. For example, the porosity of the stretched PP membrane decreased from 5% at a tensile strain of 100% to 3.1% at a tensile strain of 200%. For the stretched PP/GO membrane, the porosity increases gradually with increasing tensile strain. Although the porosity of the stretched PP/OP-10 membrane also increases gradually with the increase in tensile strain, the porosity is still very low and it is even lower than that of the stretched PP/GO membrane. However, it is interesting to observe that a greatly enhanced porosity is achieved for the stretched PP/GO/OP-10 membrane at a relatively high tensile strain (100 and 200%). For example, at a tensile strain of 200%, the porosity is enhanced up to 22.1%. This is even much higher than the porosity of the stretched β-form PP membrane obtained in our previous work,25 in which the porosity was only about 11% when the membrane was prepared under completely the same tensile conditions.
 | ||
| Fig. 8 Variation of porosity versus tensile strain of different stretched membranes. The membranes were prepared at a tensile speed of 50 mm min−1. | ||
According to the principle of porosity measuring applied in this work, it can be deduced that there are several factors influencing the value of porosity, i.e. the number of pores, the pore size, the polarity of the membrane, etc. Generally speaking, the greater the number of pores in the stretched membrane, the greater the porosity. Although larger pore size facilitates the enhancement of water flux,51 it is possibly unfavorable for porosity measurements because ethanol can not be stably reserved in pores of a very large size. Increasing the polarity of the membrane enhances the wettability of the membrane, which not only increases water flux but also results in an increase in the measured porosity.52 As shown in Fig. 6, under the same tensile conditions, the stretched PP/OP-10 membrane exhibits the biggest mean pore size while the stretched PP membrane exhibits the smallest. Although the mean pore size of the PP/GO/OP-10 membrane is smaller than those of the PP/GO and PP/OP-10 membranes, it is worth noting that the polarity of the former sample is higher than those of the latter two. Specifically, at a relatively high tensile strain, the presence of many pores as well as the deformation of the PP matrix most likely results in more exposed GO particles, which further increase the polarity of the stretched membrane. This can be indirectly demonstrated by the larger porosity of the stretched PP/GO membrane as compared to the stretched PP/OP-10 membrane, although the latter membrane exhibits a larger mean pore size. Furthermore, it can be seen that in the field of view, the number of pores in the stretched PP/GO/OP-10 membrane is much larger than that in the stretched PP/OP-10 membrane. Besides acting as stress concentrators of the initial pores that induces the formation of more pores around the initial pores by changing the stress field, the debonding of GO particles from the PP matrix is possibly the other reason for the increase in the number of pores. Consequently, a greatly enhanced porosity is achieved for the stretched PP/GO/OP-10 membrane, especially at relatively high tensile strain.
3.3 Further understanding the pore formation mechanism
The introduction pores (or voids) to adjust the mechanical properties of polymer materials has been widely researched elsewhere. For example, Y. Huang et al.53 and R. Bagheri et al.54 reported that if voids of a micrometer or more in diameter were present, the toughness of the matrix could be significantly enhanced. Specifically, according to the calculation of stress intensity at the early stage of crack growth, they also proposed that the sample containing voids exhibited a greater ability to undergo plastic deformation compared with the sample without voids. In the present work, the tensile process is a quasi-static process, which is apparently different from the dynamic impact process. However, previous results have already shown that with the simultaneous addition of OP-10 and GO, the stretched PP/GO/OP-10 membrane exhibits much higher porosity at relatively high tensile strain (100 and 200%). Therefore, it is expected that the presence of initial pores, which are induced by OP-10, can induce the change of the stress field during the tensile process and then induce the formation of more pores around the initial pores. To demonstrate this, the pore morphology around initial pores was carefully characterized, and the representative images are shown in Fig. 9. It is clearly seen that besides the deformation of the initial pores, many small pores are induced in the adjacent PP matrix around the initial pores. This clearly demonstrates the promotion role of the initial pores in inducing the formation of new pores during the tensile process. | ||
| Fig. 9 SEM images showing the formation of new pores in the adjacent PP matrix around the initial pores. | ||
To further understand the pore formation behavior of the sample during the tensile process, the crystalline structure evolution of the stretched membrane was then characterized using DSC and WAXD. Fig. 10 shows the melting behaviors of representative stretched membranes obtained at a tensile strain of 100%. It can be seen that all the membranes exhibit only one endothermic peak at about 161-164 °C, indicating that only α-form PP is present in the stretched membranes. This means that there is a transition from the β-form to the α-form during the tensile process.55 Furthermore, compared with the melting behaviors of the samples shown in Fig. 4a, one can see that, except for the PP/OP-10 membrane, which exhibits a nearly invariant Tmα before and after being stretched, the stretched PP, PP/GO and PP/GO/OP-10 membranes exhibit smaller Tmα values compared with the un-stretched samples. This indicates the occurrence of lamellar separation and/or breakage during the tensile process, which results in the formation of lamellae with more defects and/or thinner lamellar thickness.56 Fig. 11 shows the WAXD profiles of the representative stretched PP/GO/OP-10 membranes obtained at different tensile strains. Compared with the WAXD profile shown in Fig. 5, which exhibits the characteristic diffraction peaks of both α- and β-form PP, there are at least two features that need to be noted. First, all the stretched PP/GO/OP-10 membranes exhibit the diffraction peaks of only α-form PP, further showing the transformation of β-form to α-form PP during the tensile process. Second, it can be seen that the stretched membranes exhibit higher Xc-WAXD values compared with the initial sample as prepared. For example, the initial sample exhibits a Xc-WAXD of 52.9%, while the stretched membranes exhibit Xc-WAXD values of 61% at a tensile strain of 50% and 68.9% at a tensile strain of 200%.The increase in crystallinity is most likely ascribed to the second crystallization of the PP matrix induced by tensile stress. In addition, the relative size of the largest dimension of crystallite is estimated based on the peak broadening associated with the (110) diffraction peak according to the Scherrer relation:57,58
 | (4) |
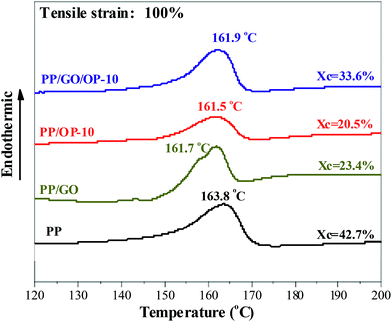 | ||
| Fig. 10 DSC heating curves showing the melting behavior of the stretched membrane. The membrane was prepared at a tensile speed of 50 mm min−1 and tensile strain of 100%. | ||
To better understand the pore formation mechanism, schematic representations are shown in Fig. 12. For the PP/OP-10 sample (Fig. 12a), during the uniaxial tensile process, the initial pores that are introduced by adding OP-10 act as a stress concentrator, which leads to a change of the stress field in the adjacent PP matrix. Therefore, the spherulites around the initial pores experience intense deformation, leading to more pore nucleation by inducing further lamellar separation and breakage, on one hand. On the other hand, because of the plasticizing effect of OP-10, the enhanced chain segment mobility of the PP matrix promotes the growth of newly-formed pores in the stress field. Consequently, a larger mean pore size is achieved for the stretched PP/OP-10 membrane, as shown in Fig. 6. In addition, the presence of a small amount of β-form PP that exhibits parallel lamellar structure is possibly one of the main reasons for the greatly increased mean pore size because the parallel lamellar structure is more easily separated compared with the cross-hatched lamellar structure of α-form PP. For the PP/GO/OP-10 sample (Fig. 12b), although the role of OP-10 is still in effect, the presence of GO particles may prevent the growth of newly-formed pores by restricting the motion of PP chain segments, which results in the formation of pores of smaller size. However, GO also acts as a stress concentrator, which results in a greater probability of lamellar separation and breakage of the adjacent PP matrix. Furthermore, the interfacial debonding that occurs between the GO and PP matrix may increase the number of pores, which facilitates the enhancement of the porosity. With the increase in tensile strain, the interfacial debonding becomes more apparent, resulting in the formation of more pores in the stretched membrane. Consequently, a higher porosity is achieved at relatively high tensile strain.
 | ||
| Fig. 12 Schematic representations showing the pore formation mechanisms in the stretched PP/OP-10 membrane (a) and stretched PP/GO/OP-10 membrane (b). | ||
This work demonstrates that the porosity of a stretched PP membrane can be greatly enhanced through simultaneous addition of pore-forming agent and GO, which endows the composite membrane with great potential for application in many fields. However, it should be stressed that only one content of OP-10 and/or GO is used in this work. Considering the apparent effect of OP-10 and/or GO on the pore formation behavior of PP membranes, studying on the effect of OP-10 and/or GO content on the pore formation becomes very significant. In addition, although the size of the pores was rigorously defined and at least 200 pores were selected during the measurements to insure that the data have statistical significance, it should be pointed out that the determination of pore size is still relatively rough. Further work needs to be done to adjust the processing parameters so that the stretched PP composite membrane can be accurately characterized through a more suitable characterization method.
4. Conclusions
Four different stretched membranes, including a PP membrane, PP/GO composite membrane, PP/OP-10 membrane and PP/GO/OP-10 composite membrane, have been prepared through a melt-compounding and subsequent tensile process. The results show that many initial pores can be introduced into the PP material by adding OP-10. The presence of OP-10 enhances the chain segment mobility but suppresses the crystallization of the PP matrix. There is a competition between OP-10 and GO particles in influencing the crystallization of the PP matrix. The presence of initial pores promotes the nucleation and growth of new pores during the tensile process, leading to a larger mean pore size for the stretched PP/OP-10 membrane. However, the presence of well-dispersed GO prevents the growth of the pores. Greatly enhanced porosity is achieved for the stretched PP/GO/OP-10 membrane, especially at relatively high tensile strain. Furthermore, increasing the tensile strain facilitates the increase of the mean pore size. Further results imply that the initial pores, which are introduced by adding OP-10, act as stress concentrators, promoting the formation of more pores during the tensile process by inducing lamellar separation and breakage.Acknowledgements
Authors express their sincere thanks to the National Natural Science Foundation of China (51203129, 51173151) for financial support. Prof. Qiang Fu (Sichuan University, P.R. China) and Dr. Xiao-tong Zheng (Southwest Jiaotong University, P.R. China) were greatly appreciated for DMA and TEM measurements, respectively.References
- H. Matsuyama, H. OIkafuji, T. Maki, M. Teramoto and N. Tsujioka, J. Appl. Polym. Sci., 2002, 84, 1701 CrossRef CAS.
- N. Tang, Q. Jia, H. J. Zhang, J. J. Li and S. Cao, Desalination, 2010, 256, 27 CrossRef CAS PubMed.
- S. Farhad, A. Ajji and P. J. Carreau, J. Membr. Sci., 2007, 292, 62 CrossRef PubMed.
- S. H. Tabatabaei, P. J. Carreau and A. Ajji, J. Membr. Sci., 2009, 345, 148 CrossRef CAS PubMed.
- F. Chu and Y. Kimur, Polymer, 1996, 37, 573 CrossRef CAS.
- G. T. Offord, S. R. Armstrong, B. D. Freeman, E. Baer, A. Hiltner, J. S. Swinnea and D. R. Paul, Polymer, 2013, 54, 2577 CrossRef CAS PubMed.
- M. B. Elias, R. Machado and S. V. Canevarolo, J. Therm. Anal. Calorim., 2000, 59, 143 CrossRef CAS.
- K. Y. Lin, M. Xanthos and K. K. Sirkar, J. Membr. Sci., 2009, 330, 267 CrossRef CAS PubMed.
- H. Matsuyama, T. Maki, M. Teramoto and K. Asano, J. Membr. Sci., 2002, 204, 323 CrossRef CAS.
- G. T. Offord, S. R. Armstrong, B. D. Freeman, E. Baer, A. Hiltner and D. R. Paul, Polymer, 2013, 54, 2796 CrossRef CAS PubMed.
- S. H. Tabatabaei, P. J. Carreau and A. Ajji, Polymer, 2009, 50, 4228 CrossRef CAS PubMed.
- C. H. Lei, S. Q. Wu, Q. Cai, R. J. Xu, B. Hu and W. Q. Shi, Polym. Int., 2014, 63, 584 CrossRef CAS.
- G. K. Elyashevich, I. S. Kuryndin, V. K. Lavrentyev, A. Y. Bobrovsky and V. Bukošek, Polymers, 2012, 54, 1907 CAS.
- M. A. Rafiee, J. Rafiee, Z. Wang, H. H. Song, Z. Z. Yu and N. Koratkar, ACS Nano, 2009, 3, 3884 CrossRef CAS PubMed.
- C. A. Crock, A. R. Rogensues, W. Q. Shan and V. V. Tarabara, Water Res., 2013, 47, 3984 CrossRef CAS PubMed.
- H. M. Sun, L. Y. Cao and L. H. Lu, Nano Res., 2011, 4, 550 CrossRef CAS PubMed.
- G. K. Ramesha, A. V. Kumara, H. B. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270 CrossRef CAS PubMed.
- W. J. Zhang, C. J. Zhou, W. C. Zhou, A. H. Lei, Q. L. Zhang, Q. Wan and B. S. Zou, Bull. Environ. Contam. Toxicol., 2011, 87, 86 CrossRef CAS PubMed.
- Z. H. Wang, H. R. Yu, J. F. Xia, F. F. Zhang, F. Li, Y. Z. Xia and Y. H. Li, Desalination, 2012, 299, 50 CrossRef CAS PubMed.
- J. G. Zhang, Z. W. Xu, M. J. Shan, B. M. Zhou, Y. L. Li, B. D. Li, J. R. Niu and X. M. Qian, J. Membr. Sci., 2013, 448, 81 CrossRef CAS PubMed.
- C. Q. Zhao, X. C. Xu, J. Chen and F. L. Yang, J. Environ. Chem. Eng., 2013, 1, 349 CrossRef CAS PubMed.
- S. Zinadini, A. A. Zinatizadeh, M. Rahimi, V. Vatanpour and H. Zangeneh, J. Membr. Sci., 2014, 453, 292 CrossRef CAS PubMed.
- H. G. Xu, H. Dai and G. N. Chen, Talanta, 2010, 81, 334 CrossRef CAS PubMed.
- S. Ramasundaram, J. H. Jung, E. Chung, S. K. Maeng, S. H. Lee, K. G. Song and S. W. Hong, Carbon, 2014, 70, 179 CrossRef CAS PubMed.
- J. Dai, X. H. Liu, J. H. Yang, N. Zhang, T. Huang, Y. Wang and Z. W. Zhou, Compos. Sci. Technol., 2014, 99, 59 CrossRef CAS PubMed.
- S. F. Ran and M. Xu, Chin. J. Polym. Sci., 2004, 22, 123 CAS.
- J. P. G. Villaluenga, M. Khayet, M. A. López-Manchado, J. L. Valentin, B. Seoane and J. I. Mengual, Eur. Polym. J., 2007, 43, 1132 CrossRef CAS PubMed.
- S. H. Tabatabaei, P. J. Carreau and A. Ajji, J. Membr. Sci., 2008, 325, 772 CrossRef CAS PubMed.
- X. M. Zhang and A. Ajji, Polymer, 2005, 46, 3385 CrossRef CAS PubMed.
- Y. D. Lv, Y. J. Huang, M. Q. Kong and G. X. Li, Polym. Test., 2013, 32, 179 CrossRef CAS PubMed.
- B. Gu, Q. G. Du and Y. L. Yang, J. Membr. Sci., 2000, 164, 59 CrossRef CAS.
- C. Chandavasu, M. Xanthos, K. K. Sirkar and C. G. Gogos, Polymer, 2002, 43, 781 CrossRef CAS.
- G. Chen, Y. K. Lin and X. L. Wang, J. Appl. Polym. Sci., 2007, 105, 2000 CrossRef CAS.
- Y. K. Lin, G. Chen, J. Yang and X. L. Wang, Desalination, 2009, 236, 8 CrossRef CAS PubMed.
- H. O. Matsuyama, H. Okafuji, T. Maki, M. Teramoto and N. Tsujioka, J. Appl. Polym. Sci., 2002, 84, 1701 CrossRef CAS.
- S. S. Kim and D. R. Lloyd, J. Membr. Sci., 1991, 64, 13 CrossRef CAS.
- W. Zhao, Y. L. Su, C. Li, Q. Shi, X. Ning and Z. Y. Jiang, J. Membr. Sci., 2008, 318, 405 CrossRef CAS PubMed.
- M. M. Meier, L. A. Kanis and V. Soldi, Int. J. Pharm., 2004, 278, 99 CrossRef CAS PubMed.
- C. L. Bashford, G. M. Alder, J. M. Graham, G. Menestrina and C. A. Pasternak, J. Membr. Biol., 1988, 103, 79 CrossRef CAS.
- J. W. Chen, J. Dai, J. H. Yang, N. Zhang, T. Huang, Y. Wang and C. L. Zhang, Ind. Eng. Chem. Res., 2014, 53, 4679 CrossRef CAS.
- S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- J. H. Yang, C. X. Feng, J. Dai, N. Zhang, T. Huang and Y. Wang, Polym. Int., 2013, 62, 1085 CAS.
- J. Li, W. Cheung and D. Jia, Polymer, 1999, 40, 1219 CrossRef CAS.
- S. J. Liu, C. X. Zhou and W. Yu, J. Membr. Sci., 2011, 379, 268 CrossRef CAS PubMed.
- J. R. Potts, D. R. Dreyer, C. W. Bielawski and R. S. Ruoff, Polymer, 2011, 52, 5 CrossRef CAS PubMed.
- C. Vallés, I. A. Kinloch, R. J. Young, N. R. Wilson and J. P. Rourke, Compos. Sci. Technol., 2013, 88, 158 CrossRef PubMed.
- J. Z. Xu, Y. Y. Liang, H. D. Huang, G. J. Zhong, J. Lei, C. Chen and Z. M. Li, J. Polym. Res., 2012, 19, 9975 CrossRef.
- R. Y. Bao, Z. T. Ding, Z. Y. Liu, W. Yang, B. H. Xie and M. B. Yang, Polymer, 2013, 54, 2059 Search PubMed.
- T. Wu, M. Xiang, Y. Cao, J. Kang and F. Yang, RSC Adv., 2014, 4, 36689 RSC.
- T. Wu, M. Xiang, Y. Cao, J. Kang and F. Yang, RSC Adv., 2014, 4, 43012 RSC.
- B. T. Kim, K. Song and S. S. Kim, Macromol. Res., 2002, 10, 127 CrossRef CAS.
- S. C. Yu, Y. P. Zheng, Q. Zhou, S. Shuai, Z. H. Lv and C. J. Gao, Desalination, 2012, 298, 49 CrossRef CAS PubMed.
- Y. Huang and A. J. Kinloch, Polymer, 1992, 33, 1330 CrossRef CAS.
- R. Bagheri and R. A. Pearson, Polymer, 1996, 37, 4529 CrossRef CAS.
- X. X. Li, H. Y. Wu, T. Huang, Y. Y. Shi, Y. Wang and F. M. Xiang, Colloid Polym. Sci., 2010, 288, 1539 CAS.
- F. Zuo, J. K. Keum, X. M. Chen, B. S. Hsiao, H. Y. Chen, S. Y. Lai, R. Wevers and J. Li, Polymer, 2007, 48, 6867 CrossRef CAS PubMed.
- F. Chu, T. Yamaoka, H. Ide and Y. Kimura, Polymer, 1994, 35, 3442 CrossRef CAS.
- J. Kotek, M. Raab, J. Baldrian and W. Grellmann, J. Appl. Polym. Sci., 2002, 85, 1174 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |