Green mechanical activation-assisted solid phase synthesis of cellulose esters using a co-reactant: effect of chain length of fatty acids on reaction efficiency and structure properties of products†
Abstract
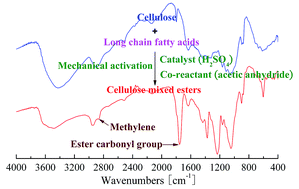
Esterification is an important chemical modification for the preparation of natural cellulose-based materials. However, esterification of cellulose is commonly carried out in organic solvents, which reduces the economic and environmental feasibility of the synthetic technologies. Herein we report a novel technology for the production of cellulose esters, which combines mechanical activation (MA) and esterification in a stirring ball mill under solid phase conditions without the use of solvents. With the use of acetic anhydride as co-reactant and fatty acids as long chain esterifying agents, 1H and 13C NMR measurements confirmed that both acetyl and long chain fatty acyl groups were successfully grafted on cellulose by the technology of MA-assisted solid phase synthesis (MASPS). The factors which contributed to the successful preparation of cellulose esters were: the formation of highly reactive mixed acetic-long chain fatty acid anhydride, the generation of active hydroxyl groups in cellulose, the weakening of the steric effect of long chain fatty acids, and the improved contact between reagents and cellulose which were first induced by intense milling. It also showed that the reactivity of fatty acids and the degree of substitution (DS) of fatty acyls decreased with the increase in their chain length, and the long chain fatty acylium ions preferred to react with the more reactive hydroxyl group in the anhydro glucose unit of cellulose. Moreover, Fourier transform infrared spectroscopy, X-ray diffractometry, and scanning electron microscopy analyses were used to measure the changes in chemical structure, crystal structure, and surface morphology of the cellulose before and after esterification by MASPS, respectively. The results indicate that this green, simple, and efficient technology is suitable for the direct production of cellulose esters with long chain substituents.

 Please wait while we load your content...
Please wait while we load your content...