Synthesis of monodispersed YbF3:Er3+ nanoplates with rhombus shapes†
Shengtai He*,
Xiaoqin Hou,
Jun Zhou,
Yanan Li and
Qin Li
School of Materials Science and Engineering, Tianjin Polytechnic University, Tianjin 300387, China. E-mail: sht-he@tjpu.edu.cn; Fax: +86 22 8395 5055; Tel: +86 22 8395 5360
First published on 6th January 2015
Abstract
Novel monodispersed YbF3:Er3+ nanoplates with rhombus shapes are synthesized via a thermolysis process in this study. The morphology and luminescence properties have been investigated using transmission electron microscopy (TEM) and emission spectroscopy.
Controlling inorganic nanocrystals (NCs) with well-defined shapes and architectures is always an important route to finely tune their properties, because the properties of the materials closely relate to geometrical factors such as morphology, dimensionality, and size.1–3 In the past decade, lots of effort has been made to fabricate a variety of inorganic crystals to enhance their performance. Among them, the wet chemical method has been proved to be one of the most effective and convenient approaches in preparing various inorganic materials with diverse controllable morphologies and architectures in terms of low cost and large scale production.
In recent years, research on lanthanide (Ln3+) ions doped fluoride crystals is rapidly increasing due to their wide applications in solar cell, flat panel display, fluorescence imaging, drug deliver.4–8 The Ln3+ ions can undergo a process known as up-conversion (UC) in which they convert low energy radiation such as near infrared (NIR) or IR light to high energy radiation including visible or ultraviolet (UV) light.9,10 Typically in UC fluoride crystals, hexagonal (β) phase NaREF4 (RE = rare earth ions) crystals are identified as the ideal UC luminescence matrices owing to their low phonon energy, high Ln3+ ion solubility and high photochemical stability.11–13 Besides β-NaREF4, orthorhombic REF3 crystals have been proved to be another efficient high order UC luminescence materials.14–16 Furthermore, YbF3:Er shows strong down-conversion (DC) properties when excited with UV light.17,18
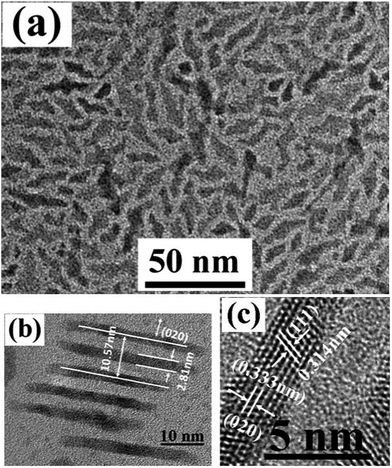
Here, Er3+ doped YbF3 rhombus nanoplates (NPs) are first synthesized with a high temperature thermolysis process. Fig. 1a presents a TEM image of as-synthesized YbF3:10% Er3+ NPs. It is found that almost all the nanoplates show clear rhombus shape and size of as-synthesized NPs is well dispersed. The length and width of NPs are 12.2 ± 2.1 nm and 10.9 ± 1.8 nm, respectively. The corresponding XRD and electron diffraction patterns (shown as S4 and S5†) demonstrate that as-synthesized nanoplate is YbF3 {Pnma} orthorhombic crystal structure. The diffraction band of as-synthesized nanoplates is consistent with the JCPDS 49-1805 file of crystal YbF3. Furthermore, from the high resolution TEM image of Fig. 1b, well crystalline lattice structures can be observed. Most of nanoplates grow along (101) with a lattice distance of 0.360 nm. The shape of YbF3 nanoplate can be affected by the amount of Er3+ and oleic acid. Fig. 2a presents a TEM image of YbF3:50% Er3+ nanoplates. As shown in Fig. 2a, instead of well disperse single rhombus plate, short belts consisted by 2–4 single rhombus plates are observed for YbF3:50% Er3+ plate. Fig. 2b is a HRTEM image of an aligned YbF3:50% Er3+ plate standing on the edge array. The distance between two standing plate is about 2.5 nm, and the thickness of nanoplate is ca. 2.8 nm. The analysis results from HRTEM image of plate edge demonstrate that the edge grow along (111) and (020) directions. While, when the amount of Er3+ is up to 90%, as-synthesized nanoplates are well disperse single rhombus plates (see S9†). Furthermore, the shape or structure can be affected by the amount of oleic acid. For example, for YbF3:10% Er3+ discussed above, with same synthesis conditions, when volume of oleic acid is reduced from 3.2 ml to 1.6 ml, similar structure to that of YbF3: 50% Er3+ nanoplate are also observed. Currently, we attribute the formation of short belt of YbF3:10% to the overgrowth of nanoplate. With less oleic acid, several nanoplates grow and band together to form large nanoplate belt. Further experiments and analyses are being done to explore the effect of Er3+ amount and oleic acid on the structure of Er3+ doped YbF3 nanoparticles.
For the photovoltaic efficiency of semiconductor solar cells, the thermalization and sub-band gap losses with UV and NIR are the major bottleneck. Up-conversion can generate one high energy photon from two or more incident low energy (sub-band gap) photons, whereas the down-conversion can generate more than one low-energy (sub-band gap) photons for each incident high-energy photon. In this communication, we report the synergistic effect of UV down-conversion and NIR up-conversion of as-synthesized YbF3:10% Er3+ nanoplates.
Fig. 3 presents the absorption and emission spectra of as-synthesized YbF3:10% Er3+ NPs. It is well known that (Er3+, Yb3+) is one of the efficient up-conversion couples. Efficient up-conversion has been reported for this couple in many host lattices and it is used in the efficient detection of near 1000 nm IR radiation. After excitation to the 2F5/2 level of Yb3+, two sequential energy transfer steps excite the Er3+ ion from the 4I15/2 ground state to the 4I11/2 excited state and from the 4I11/2 excited state to the higher energy 4F7/2 excited state. Visible emission may be observed from the 4F7/2 state or, after relaxation, from the lower energy 2H11/2 and 4S3/2 states. Fig. 3b is the UC emission spectra of as-synthesized YbF3:10% Er3+ NPs upon an excitation wavelength of 980 nm. The weak violet emission centred at 430 nm is attributed to the 2H9/2–4I15/2 transition of Er3+ ions. The obvious green emission peak at 542 nm is assigned to the 4S3/2–4I15/2 transition of the Er3+ ions, while the red emission peak at 610 nm is attributed to the Er3+ 4F9/2–4I15/2 transition. Furthermore, (Er3+, Yb3+) couple is an attractive DC materials for use in combination with c-Si solar cells.19–21 The choice of Yb3+ as the emitting acceptor is due to the favourable energy of the 2F5/2 excited state and the fact that the Yb3+ has no other 4f excited states that can interfere with the down-conversion process. Fig. 3c shows a DC emission spectrum of as synthesized YbF3:10% Er3+ NPs excited at 369 nm. The strong emission at 480 nm is attributed to the 4F7/2–4I15/2 transition of the Er3+ ions.
 | ||
| Fig. 3 Absorption spectrum (a), up-conversion (b) and down-conversion (c) Emission of as-synthesized YbF3:10% Er3+ nanoplates (in b, the laser power of 980 nm laser diode is 1 W). | ||
Conclusions
In this study, monodisperse Er3+ doped YbF3 nanoplates with regular rhombus shape are synthesized by a thermolysis process. The as-synthesized YbF3:10% Er3+ nanoplates are small and its size is less than 15 nm. The thickness of plate is about 2.8 nm. All the nanoplates are well dispersed and have a good crystalline. The analysis results demonstrate that the structure of plate can be affected by the amounts of doped Er3+ ion and oleic acid reagent. Moreover, the as-synthesized YbF3:10% Er3+ nanoplates show strong up-conversion and down-conversion emission properties.Acknowledgements
This work is funded by National Key Basic Research Program (973 Program) of China (no. 2012CB933301) and Tianjin Research Program of Applied Basic & Cutting-edge Technologies (no. 09JCYBJC27200).Notes and references
- Y. Xia, Y. Xiong, B. Lim and S. Skrabalak, Angew. Chem., Int. Ed., 2009, 49, 60 CrossRef PubMed.
- H. Chen, L. Shao, Q. Li and J. Wang, Chem. Soc. Rev., 2013, 42, 2679 RSC.
- M. A. EI-Sayed, Acc. Chem. Res., 2001, 34, 257 CrossRef PubMed.
- J. W. Wang and P. A. Tanner, J. Am. Chem. Soc., 2009, 132, 947 CrossRef PubMed.
- J. Bunzli and S. V. Lanthanide, J. Rare Earths, 2010, 28, 824 CrossRef CAS.
- M. Katkova, V. Ilichev, A. Konev, I. I. Pestova, G. Fukin and M. Bochkarev, Org. Electron., 2009, 10, 623 CrossRef CAS PubMed.
- A. Yerpude and S. Dhoble, J. Lumin., 2012, 132, 1781 CrossRef CAS PubMed.
- F. Wang, Y. Han, C. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- F. Auzel, Chem. Rev., 2003, 104, 139 CrossRef PubMed.
- H. J. Wu, Z. W. Yang, J. Y. Liao, S. F. Lai, J. B. Qiu, Z. G. Song, Y. Yang, D. C. Zhou and Z. Y. Yin, J. Alloys Compd., 2014, 586, 485 CrossRef CAS PubMed.
- Z. Wang, C. H. Liu, L. J. Chang and Z. P. Li, J. Mater. Chem., 2012, 22, 12186 RSC.
- T. Jiang, Y. Liu, S. S. Liu, N. Liu and W. P. Qin, J. Colloid Interface Sci., 2012, 377, 81 CrossRef CAS PubMed.
- Z. G. Yi, G. Z. Ren, L. Rao, H. B. Wang, H. B. Wang, H. R. Liu and S. J. Zeng, J. Alloys Compd., 2014, 589, 502 CrossRef CAS PubMed.
- D. L. Gao, X. Y. Zhang, H. R. Zheng, W. Gao and E. J. He, J. Alloys Compd., 2013, 554, 395 CrossRef CAS PubMed.
- C. H. Liu, H. Wang, X. Li and D. Chen, J. Mater. Chem., 2009, 19, 3546 RSC.
- G. S. Yi and G. M. Chow, Adv. Funct. Mater., 2006, 16, 2324 CrossRef CAS.
- X. F. Wang and X. H. Yan, Opt. Lett., 2011, 36, 4353 CrossRef CAS PubMed.
- Y. Zhang, R. Wang, S. Xiao, M. Qu, K. Li, C. Liu, X. Wang, X. Yan and H. Yan, J. Lumin., 2014, 145, 351 CrossRef CAS PubMed.
- P. Vergeer, T. J. H. Vlugt, M. H. F. Kox, M. I. Den Hertog, J. P. J. M. Van der Eerden and A. Meljerink, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 014119 CrossRef.
- L. Aarts, B. M. van der Ende and A. Maljerink, J. Appl. Phys., 2009, 106, 023522 CrossRef PubMed.
- A. Wang and X. Yan, Opt. Lett., 2011, 36, 4353 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed materials synthesis method and characterization data. See DOI: 10.1039/c4ra13271h |
| This journal is © The Royal Society of Chemistry 2015 |