Photophysical and thermal properties of novel solid state fluorescent benzoxazole based styryl dyes from a DFT study†
Urmiladevi Narad Yadava,
Haribhau Shantaram Kumbhara,
Saurabh Satish Deshpandea,
Suban Kumar Sahoob and
Ganapati Subray Shankarling*a
aDyestuff Technology Department, Institute of Chemical Technology, Matunga, Mumbai 400 019, India. E-mail: gsshankarling@gmail.com; gs.shankarling@ictmumbai.edu.in; Fax: +91 22 33611020; Tel: +91 22 33612708
bDepartment of Applied Chemistry, SV National Institute of Technology (SVNIT), Surat-395007, India. E-mail: suban_sahoo@rediffmail.com
First published on 7th May 2015
Abstract
Novel benzoxazole styryl dyes 6a–6e with donor(D)–π(pi)–acceptor(A) were synthesized and characterized. The spectral (absorption and emission) and thermogravimetric properties of the dyes were investigated. They were found to possess red-shifted absorption with a high molar extinction coefficient when compared to their reported analogues. The compounds exhibited enhanced fluorescence emission in the solid state (540–603 nm). They showed excellent thermal and photochemical stability. The effect of the benzoxazole moiety on the photophysical properties is explained by comparing with reported analogues and density functional theory (DFT) calculations. Thus investigations of photophysical properties provide an important foundation for the molecular design and development of novel optoelectronic materials for OLED and NLO applications.
Introduction
Fluorescent styryl dyes have attracted researchers all over the globe for their applications in potentially interesting fields such as organic light-emitting diodes (OLEDs),1 solar cells,2 fluorescent viscosity sensors,3 molecular probes,4 non-linear optics,5 laser dyes,6 fluorescent thermometers,7 etc. They have great commercial demand since they are easily tailor-made depending on the requirements. Styryl dyes based on benzoxazole have been exploited for their excellent luminescent properties. They have been known to exhibit strong photoluminescence (PL) in the blue region.8,9 Styryl dyes with benzoxazole as an acceptor have been used as an emitter material in organic electroluminescent (EL) devices.10 Intramolecular exciplexes based on benzoxazole have been applied as fluorescent sensors for cations.11 Derivatives of 2-hydroxy phenyl benzoxazole form borate complexes (BODIPY) which are highly luminescent12 and useful in monitoring of polymerization process.13 Benzoxazole is widely employed as a key structure in many fields, i.e. ESIPT dyes14–16 or metal cation indicators.17The reason for enhanced fluorescence on introduction of benzoxazolyl group in stilbene can be attributed to the decreased non-radiative processes due to the higher resistance to rotation offered by the bulky benzoxazolyl molecule while radiative process suffer competition from well-known trans–cis isomerisation.18 Considering the effects of benzoxazole moiety on enhanced fluorescence of stilbenes, novel styryl dyes containing benzoxazole were synthesized (Scheme 1) and their photophysical properties both in the solution as well as in solid states were investigated. The effect of solvent polarity on the absorption and emission spectra were evaluated. Thermal stability of these synthesized dyes was also explored. The comparative study of the photophysical properties of synthesized dyes with other reported analogues highlighted the superiority in optical properties. One of the added advantages of the system is the combination of a weak donor (benzoxazole) and a very strong acceptor (CN) end groups, which makes this type of compound suitable for non-linear optical applications owing to non-linear transparency.19
Experimental section
Materials and methods
All common reagent grade chemicals were procured from SD Fine Chemical Ltd. (Mumbai, India) and were used without further purification. The reactions were monitored by thin-layered chromatography (TLC) using 0.25 mm E-Merck silica gel 60 F254 precoated plates, which were visualized with UV light. 1H NMR spectra were recorded on a 400 MHz Varian mercury plus spectrometer. Chemical shifts were expressed in δ ppm using TMS as an internal standard. Mass spectra were obtained with a micromass-Q-TOF (YA105) spectrometer. All the DSC-TGA measurements were performed out on a SDT Q600 v8.2 Build 100 model of TA instruments Waters (India) Pvt. Ltd. Absorption spectra were recorded on a Perkin Elmer Lamda 25 UV-VIS Spectrophotometer. Fluorescence spectra were recorded on a Varian Cary Eclipse fluorescence Spectrophotometer using freshly prepared solutions of 1 × 10−5 M. Absorption and emission spectra were performed using quartz cell of 1 cm path length. All compounds were excited at their maximum absorption values. All the experimental parameters were kept constant throughout in order to have precision and accuracy. Absorption and emission graphs were plotted using MATLAB R 2008a.Ink software.Synthesis of the dyes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate (9
ethylacetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent system. Yield: 62%. FT-IR (KBr, cm−1): 2953 (C–H, Ar), 2217 (CN), 1560 (C
1) as eluent system. Yield: 62%. FT-IR (KBr, cm−1): 2953 (C–H, Ar), 2217 (CN), 1560 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). 1H-NMR (DMSO-d6, 400 MHz): δ = 1.03 (s, 6H, –2CH3), 2.58 (s, 2H, –CH2), 2.64 (s, 2H, –CH2), 6.98 (s, 1H, vinylic–H), 7.38 (d, J = 16.4 Hz, 1H), 7.44 (t, J = 6.0, 11.2 Hz, 2H), 7.62 (d, J = 16.0 Hz, 1H), 7.82 (t, J = 8.8, 16.8 Hz, 2H), 7.94 (d, J = 8.4 Hz, 2H), 8.22 (d, J = 8.4 Hz, 2H). 13C-NMR (CDCl3, 126 MHz): δ = 28.0, 32.0, 39.2, 42.9, 79.6, 110.6, 112.4, 113.2, 120.1, 124.5, 124.7, 125.4, 127.8, 127.9, 128.1, 130.8, 135.5, 138.5, 142.1, 150.7, 153.0, 162.3, 168.9. HRMS (m/z): calcd for C26H21N3O ([M + H])+ 392.1763, observed 392.1721.
C). 1H-NMR (DMSO-d6, 400 MHz): δ = 1.03 (s, 6H, –2CH3), 2.58 (s, 2H, –CH2), 2.64 (s, 2H, –CH2), 6.98 (s, 1H, vinylic–H), 7.38 (d, J = 16.4 Hz, 1H), 7.44 (t, J = 6.0, 11.2 Hz, 2H), 7.62 (d, J = 16.0 Hz, 1H), 7.82 (t, J = 8.8, 16.8 Hz, 2H), 7.94 (d, J = 8.4 Hz, 2H), 8.22 (d, J = 8.4 Hz, 2H). 13C-NMR (CDCl3, 126 MHz): δ = 28.0, 32.0, 39.2, 42.9, 79.6, 110.6, 112.4, 113.2, 120.1, 124.5, 124.7, 125.4, 127.8, 127.9, 128.1, 130.8, 135.5, 138.5, 142.1, 150.7, 153.0, 162.3, 168.9. HRMS (m/z): calcd for C26H21N3O ([M + H])+ 392.1763, observed 392.1721.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate (8
ethylacetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent system. Yield: 67%. FT-IR (KBr, cm−1): 3461, 3334 (NH2), 3155, 2960, 2343 (CN), 1662 (C
2) as eluent system. Yield: 67%. FT-IR (KBr, cm−1): 3461, 3334 (NH2), 3155, 2960, 2343 (CN), 1662 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1514 (C–O), 1401 (C–N). 1H-NMR (DMSO-d6, 400 MHz): δ = 0.98 (s, 6H, –2CH3), 2.51 (s, 2H, –CH2), 2.60 (s, 2H, –CH2), 6.84 (s, 1H), 7.11 (d, J = 16.4 Hz, 1H), 7.41 (d, J = 7.6 Hz, 2H), 7.45 (d, J = 16.4 Hz, 1H), 7.67 (s, 1H), 7.79–7.84 (m, 4H, ArH), 7.88 (d, J = 8.4 Hz, 2H), 8.18 (d, J = 8.4 Hz, 2H). 13C-NMR (DMSO-d6, 100 MHz): δ = 27.8, 30.77, 31.06, 104.5, 110.8, 116.71, 119.7, 124.9, 125.27, 125.58, 125.8, 126.2, 127.5, 127.9, 128.17, 128.8, 131.9, 132.4, 139.8, 141.5, 148.4, 150.1, 157.5, 161.9, 163.4. HRMS (m/z): calcd for C26H23N3O2 ([M + H])+ 410.1869, observed 410.1861.
O), 1514 (C–O), 1401 (C–N). 1H-NMR (DMSO-d6, 400 MHz): δ = 0.98 (s, 6H, –2CH3), 2.51 (s, 2H, –CH2), 2.60 (s, 2H, –CH2), 6.84 (s, 1H), 7.11 (d, J = 16.4 Hz, 1H), 7.41 (d, J = 7.6 Hz, 2H), 7.45 (d, J = 16.4 Hz, 1H), 7.67 (s, 1H), 7.79–7.84 (m, 4H, ArH), 7.88 (d, J = 8.4 Hz, 2H), 8.18 (d, J = 8.4 Hz, 2H). 13C-NMR (DMSO-d6, 100 MHz): δ = 27.8, 30.77, 31.06, 104.5, 110.8, 116.71, 119.7, 124.9, 125.27, 125.58, 125.8, 126.2, 127.5, 127.9, 128.17, 128.8, 131.9, 132.4, 139.8, 141.5, 148.4, 150.1, 157.5, 161.9, 163.4. HRMS (m/z): calcd for C26H23N3O2 ([M + H])+ 410.1869, observed 410.1861.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (8
ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent system. Yield: 48%. FT-IR (KBr, cm−1): 2960, 2343, 2209 (CN), 1712 (C
2) as eluent system. Yield: 48%. FT-IR (KBr, cm−1): 2960, 2343, 2209 (CN), 1712 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1526 (C
O), 1526 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1232 (C–O). 1H-NMR (DMSO-d6, 400 MHz): δ = 1.03 (s, 6H, –2CH3), 1.28 (t, 3H, –CH3), 2.61 (s, 2H, –CH2), 2.94 (s, 2H, –CH2), 4.25 (q, 2H, –CH2), 6.96 (s, 1H, vinylic–H), 7.26 (d, J = 16.0 Hz, 1H), 7.36–7.45 (m, 3H, ArH), 7.52 (d, J = 16.0 Hz, 1H), 7.81 (t, J = 8.8, 17.6 Hz, 2H), 7.92 (d, J = 8.4 Hz, 2H), 8.19 (d, J = 8.4 Hz, 2H). 13C-NMR (DMSO-d6, 100 MHz): δ = 13.9, 14.0, 27.6, 27.8, 30.6, 31.0, 31.4, 61.2, 110.9, 115.9, 119.8, 124.6, 124.9, 125.6, 126.2, 126.6, 127.6, 128.2, 132.1, 132.4, 133.9, 134.1, 141.5, 150.2, 152.1, 152.9, 161.8, 162.2. HRMS (m/z): calcd for C28H26N2O3 ([M + H])+ 439.2022, observed 439.1988.
C), 1232 (C–O). 1H-NMR (DMSO-d6, 400 MHz): δ = 1.03 (s, 6H, –2CH3), 1.28 (t, 3H, –CH3), 2.61 (s, 2H, –CH2), 2.94 (s, 2H, –CH2), 4.25 (q, 2H, –CH2), 6.96 (s, 1H, vinylic–H), 7.26 (d, J = 16.0 Hz, 1H), 7.36–7.45 (m, 3H, ArH), 7.52 (d, J = 16.0 Hz, 1H), 7.81 (t, J = 8.8, 17.6 Hz, 2H), 7.92 (d, J = 8.4 Hz, 2H), 8.19 (d, J = 8.4 Hz, 2H). 13C-NMR (DMSO-d6, 100 MHz): δ = 13.9, 14.0, 27.6, 27.8, 30.6, 31.0, 31.4, 61.2, 110.9, 115.9, 119.8, 124.6, 124.9, 125.6, 126.2, 126.6, 127.6, 128.2, 132.1, 132.4, 133.9, 134.1, 141.5, 150.2, 152.1, 152.9, 161.8, 162.2. HRMS (m/z): calcd for C28H26N2O3 ([M + H])+ 439.2022, observed 439.1988.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate (8
ethylacetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent system. Yield: 59%. FT-IR (KBr, cm−1): 3047 (C–H, Ar), 2343 (CN), 1681 (C
2) as eluent system. Yield: 59%. FT-IR (KBr, cm−1): 3047 (C–H, Ar), 2343 (CN), 1681 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1450 (C–N), 1241, 1059, 759, 743. 1H NMR (500 MHz, CDCl3) 7.36–7.44 (m, 2H), 7.46 (t, J = 7.1 Hz, 1H), 7.56 (t, J = 8.2 Hz, 1H), 7.60–7.69 (m, 1H), 7.79–7.85 (m, 1H), 7.94 (dd, J = 8.0, 0.5 Hz, 1H), 8.11 (dd, J = 8.2, 0.5 Hz, 1H), 8.18 (d, J = 8.7 Hz, 2H), 8.29 (s, 1H), δ 8.39 (d, J = 8.5 Hz, 2H). 13C NMR (126 MHz, CDCl3) 106.94, 110.81, 116.24, 120.39, 121.74, 123.77, 124.95, 125.86, 126.26, 127.10, 128.18, 130.04, 130.76, 134.85, 135.08, 142.05, 145.22, 150.87, 153.59, 161.69, δ 162.27. HRMS (m/z): calcd for C23H13N3OS ([M + H])+ 380.0858, observed 380.0826.
O), 1450 (C–N), 1241, 1059, 759, 743. 1H NMR (500 MHz, CDCl3) 7.36–7.44 (m, 2H), 7.46 (t, J = 7.1 Hz, 1H), 7.56 (t, J = 8.2 Hz, 1H), 7.60–7.69 (m, 1H), 7.79–7.85 (m, 1H), 7.94 (dd, J = 8.0, 0.5 Hz, 1H), 8.11 (dd, J = 8.2, 0.5 Hz, 1H), 8.18 (d, J = 8.7 Hz, 2H), 8.29 (s, 1H), δ 8.39 (d, J = 8.5 Hz, 2H). 13C NMR (126 MHz, CDCl3) 106.94, 110.81, 116.24, 120.39, 121.74, 123.77, 124.95, 125.86, 126.26, 127.10, 128.18, 130.04, 130.76, 134.85, 135.08, 142.05, 145.22, 150.87, 153.59, 161.69, δ 162.27. HRMS (m/z): calcd for C23H13N3OS ([M + H])+ 380.0858, observed 380.0826.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate (9
ethylacetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent system. Yield: 64%. FT-IR (KBr, cm−1): 3285 (NH), 3051 (C–H, Ar), 2237 (CN), 1594, 1416 (C–N), 1314, 1243, 1057, 740. 1H-NMR (DMSO-d6, 400 MHz): δ = 7.27–7.32 (m, 2H), 7.37–7.45 (m, 2H), 7.63–7.68 (m, 2H), 7.77–7.81 (m, 2H), 8.17 (d, 2H, J = 8.4 Hz), 8.38 (d, 2H, J = 8.4 Hz), 8.48 (s, 1H). 13C-NMR (DMSO-d6, 126 MHz): δ = 104.4, 111.5, 116.3, 120.5, 125.5, 126.5, 128.3, 128.9, 130.7, 136.0, 141.9, 144.3, 147.6, 150.8, 161.8. HRMS (m/z): calcd for C23H14N4O ([M + H])+ 363.1203, observed 363.1181.
1) as eluent system. Yield: 64%. FT-IR (KBr, cm−1): 3285 (NH), 3051 (C–H, Ar), 2237 (CN), 1594, 1416 (C–N), 1314, 1243, 1057, 740. 1H-NMR (DMSO-d6, 400 MHz): δ = 7.27–7.32 (m, 2H), 7.37–7.45 (m, 2H), 7.63–7.68 (m, 2H), 7.77–7.81 (m, 2H), 8.17 (d, 2H, J = 8.4 Hz), 8.38 (d, 2H, J = 8.4 Hz), 8.48 (s, 1H). 13C-NMR (DMSO-d6, 126 MHz): δ = 104.4, 111.5, 116.3, 120.5, 125.5, 126.5, 128.3, 128.9, 130.7, 136.0, 141.9, 144.3, 147.6, 150.8, 161.8. HRMS (m/z): calcd for C23H14N4O ([M + H])+ 363.1203, observed 363.1181.Results and discussion
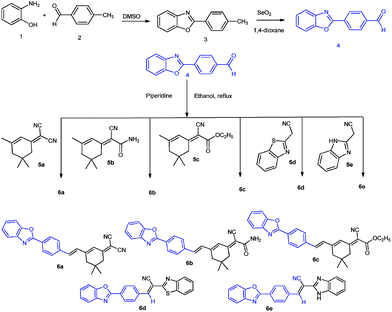
Synthetic strategy of dyes 6a–6e
Dyes 6a–6e were synthesized by classical Knovenaegel condensation of 4-(benzo[d]oxazol-2-yl)benzaldehyde with different acceptor chromophoric systems based on substituted isophorone, benzothiazole and benzimidazole. (Benzo[d]oxazol-2-yl)benzaldehyde (4) was prepared by oxidation (selenium dioxide in dioxane) of 2-(p-tolyl)benzo[d]oxazole (3), obtained by condensation of ortho-aminophenol (1) with tolualdehyde (2). The aldehyde (4) after conventional condensation with different active methylenes (5a–5e) in ethanol-piperidine gave the target styryl dyes (6a–6e). The structures and purities of the prepared compounds were confirmed using FTIR, 1H NMR, ESI-MS spectrometry and thin layer chromatography. The spectral data are in good agreement with the expected structure of the compounds and the data of analysis is provided in the experimental section. It is noteworthy that the 1H NMR spectra of dyes 6a–6c, bearing vinyl hydrogens display two characteristic doublets localized at chemical shifts in the range of 6–8 ppm. Based on the large coupling values (J) of 16–17 Hz for the olefinic protons it is established that these dyes exist in trans-conformation in the ground state.UV-vis absorption and emission of dyes 6a–6e
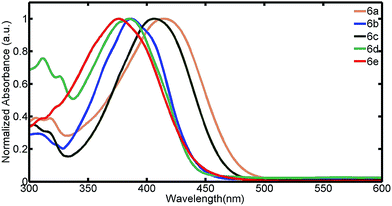
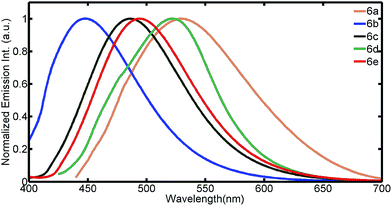
The photophysical properties of the dye were investigated in DMSO solvent (Fig. 1 and 2). All the dyes displayed an intramolecular charge transfer (ICT) band arising due to the presence of benzoxazole moiety as donor in the presence of strong acceptor system (CN) which is in agreement with DFT study. Inspection of absorption spectra reveals that the intensity and position of charge transfer (CT) band is dependent on the type of acceptor heterocyclic system. Dyes 6a, 6b and 6c showed an absorbance band at 415 nm, 387 nm and 407 nm respectively. The hypsochromic shift of 6b is due to weaker pulling strength of acceptor cyano-acetamide as compared with malononitrile (6a) and ethylcyanoacetate (6c). Compounds 6d and 6e absorbed at 383 nm and 377 nm respectively. Thus a “blue-shift” is observed in absorption spectra of compounds on going from 6a–6e, due to weaker push–pull effect.The fluorescence emissions of the compounds were studied (Fig. 2). Dye 6a exhibited good Stokes shift of 123 nm followed by 6e (116 nm), 6d (84 nm) while 6b and 6c gave similar shift of 55 nm.
The UV-vis absorption and emission of the compounds were studied in solvents of varying polarity, dielectric constant and refractive index as listed in Table 1. Molecules with π-electron system exhibit different charge distribution in the electronic ground state and in excited state and hence show solvatochromism, which is very-well established experimentally. All the dyes exhibited single broad absorption profile in the range of 300–600 nm. Thus, for the tested organic styryl dyes only a comparatively less solvent dependence is observed in UV-vis absorption spectra, while considerable effect is seen over fluorescence spectra. For dye 6a (Fig. S1†) with an isophorone–malononitrile acceptor system, a single broad absorption maxima at 407 nm in toluene was observed which varied to 415 nm in DMSO. Furthermore, a red-shift was observed in fluorescence spectra with maxima changing from 502 nm in toluene to 530 nm in DMSO. Thus, a remarkable solvatofluorism was observed for dye 6a with weak emission intensity which is attributed to the stabilization of excited state by solvent molecules. On excitation, intra molecular charge transfer (ICT) occurs, and the excited state gets much more dipolar than ground state. Stabilization of this dipolar state by differential solvation results in lowering of band gap giving rise to red-shifted emission.20
| Solvents | 6a | 6b | 6c | 6d | 6e | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λabs, nm (ε × 104) | λems, nm | λexct, nm | Δλ, cm−1 | λabs, nm (ε × 104) | λems, nm | λexct, nm | Δλ, cm−1 | λabs, nm (ε × 104) | λems, nm | λexct, nm | Δλ, cm−1 | λabs, nm (ε × 104) | λems, nm | λexct, nm | Δλ, cm−1 | λabs, nm (ε × 104) | λems, nm | λexct, nm | Δλ, cm−1 | |
| a Molar absorptivity (ε) in M−1 cm−1. The concentration of dyes used for recording absorbance and emission spectra was 1 × 10−5 mol L−1. | ||||||||||||||||||||
| Toluene | 407 (6.26) | 502 | 406 | 4650 | 396 (3.37) | 461 | 398 | 3561 | 401 (5.05) | 461 | 405 | 3246 | 387 (4.04) | 460 | 388 | 4101 | 386 (2.36) | 477 | 388 | 4942 |
| EDC | 408 (6.27) | 515 | 409 | 5092 | 394 (3.92) | 485 | 395 | 4762 | 403 (5.32) | 495 | 404 | 4612 | 383 (4.50) | 506 | 385 | 6347 | 412 (1.97) | 500 | 412 | 4272 |
| THF | 405 (6.43) | 520 | 411 | 5461 | 388 (3.70) | 453 | 388 | 3698 | 397 (5.53) | 450 | 397 | 2967 | 377 (3.19) | 450 | 378 | 4303 | 376 (3.19) | 477 | 378 | 5631 |
| Acetone | 403 (5.90) | 527 | 404 | 5839 | 383 (2.84) | 456 | 385 | 4180 | 395 (4.81) | 464 | 398 | 3765 | 376 (2.98) | 464 | 378 | 5044 | 374 (3.62) | 479 | 372 | 5861 |
| Ethanol | 404 (6.02) | 465 | 409 | 3247 | 382 (3.69) | 450 | 383 | 3956 | 396 (5.63) | 457 | 398 | 3371 | 377 (3.74) | 452 | 377 | 4401 | 363 (3.08) | 477 | 365 | 6584 |
| ACN | 402 (6.53) | 525 | 407 | 5828 | 382 (2.84) | 446 | 384 | 3756 | 394 (5.50) | 500 | 395 | 5381 | 377 (2.57) | 480 | 379 | 5692 | 374 (4.14) | 482 | 375 | 5991 |
| DMF | 409 (5.33) | 538 | 412 | 5863 | 383 (2.39) | 463 | 385 | 4511 | 402 (5.12) | 508 | 402 | 5191 | 384 (4.16) | 472 | 385 | 4855 | 378 (3.72) | 490 | 380 | 6047 |
| DMSO | 415 (4.73) | 530 | 418 | 5193 | 387 (3.63) | 447 | 387 | 3568 | 407 (5.26) | 486 | 409 | 3994 | 383 (1.63) | 523 | 385 | 6989 | 377 (3.04) | 494 | 378 | 6282 |
For dyes 6b and 6c bearing an isophorone–cyanoacetamide and isophorone–ethylcyanoacetate based acceptor system respectively (Fig. S2 and S3†), different absorption maxima were observed. In case of dye 6b, a hypsochromic shift was observed in both absorption and emission spectra. The absorption shifted from 396 nm in toluene to 387 nm in DMSO whereas emission value varied from 461 nm to 447 nm in DMSO. Dye 6c showed solvatofluorism with increase in solvent polarity from toluene (461 nm) to DMSO (486 nm).
Dyes 6d and 6e (Fig. S4 and S5†) with benzothiazole and benzimidazole as acceptors, were found to show positive solvatofluorism. The emission changed from 460 nm (toluene) to 523 nm (DMSO) for dye 6d and 477 nm (toluene) to 494 nm (DMSO) for dye 6e. The progressive bathochromic shift of charge-transfer band with increase in the polarity of the solvent depicts that the fluorescence originates from a highly polar state. Dye 6d was found to fluoresce intensely in DMSO indicating greater stabilization of excited state. The increase in emission intensity for dye 6d is tentatively assigned to decrease non-radiative decay channel which is enhanced by high polarity.
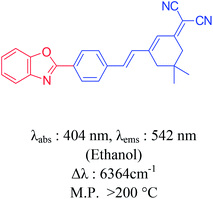
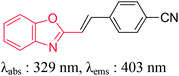
The high molar extinction coefficients values (2.48–6.93 × 104 L mol−1 cm−1) of dyes 6a–6e is due to the S0 → CT band transitions. The intramolecular charge transfer takes place through D–π–A system involving the electron flow from benzoxazole moiety towards CN groups resulting in decrease of energy gap between HOMO and LUMO. All the dyes exhibited large Stokes shift in the range of 3.25 × 103 to 6.58 × 103 cm−1. The large bathochromic shifts of the emission bands with the solvent polarity indicate greater stabilization of the excited singlet state in polar solvents. It is the characteristic for molecules which have enlarged dipoles and charge transfer (CT) characters in their excited singlet states.21 The relative fluorescence quantum yields of the dyes 6a–6e are listed in Table 2. Anthracene was used as standard for the measurement of the fluorescence quantum yield (Φf = 0.27 in ethanol). Dyes 6d and 6e displayed high quantum yield value. In addition, benzoxazolyl containing styryl dyes have higher quantum yield as compare to previously reported compound.22 A comparative study of synthesized dye molecules with reported analogues was done. It is interesting to note that the absorption and emission wavelengths of these dyes are significantly red-shifted when compared to reported analogues as listed in Table 3. The red-shifted emission is attributed to the decreased non-radiative decay processes in the excited state. The thermal stability of these compounds was remarkably higher as compared to their analogues.
| Solvent | 6a (Φf) | 6b (Φf) | 6c (Φf) | 6d (Φf) | 6e (Φf) |
|---|---|---|---|---|---|
| a The concentration of dye (C) used for calculation of quantum yield is 1 × 10−5 mol L−1. | |||||
| THF | 0.0444 | 0.0436 | 0.0303 | 0.0783 | 0.2400 |
| EDC | 0.0242 | 0.02102 | 0.0177 | 0.0285 | 0.1704 |
| Ethanol | 0.0977 | 0.0153 | 0.0115 | 0.0426 | 0.1152 |
| DMF | 0.02154 | 0.01755 | 0.01097 | 0.1036 | 0.2149 |
Solid state fluorescence
It was found that the dyes 6a–6e are highly fluorescent in solid state (Fig. 3). Intense yellowish emissions were observed upon illumination of the solid dye under UV lamp (365 nm). Materials showing fluorescence in solid state have potential application as electroluminescence devices. The solid state fluorescence emission maxima for the compounds were recorded on Varian Cary Eclipse Fluorescence Spectrophotometer. Dyes 6a–6e exhibited yellow fluorescence in solid state and their emission maxima are shown in Fig. 3. The results suggest that these solid state yellow luminescent dyes could possibly be used in electroluminescence devices, as phosphor and in organic light emitting diodes.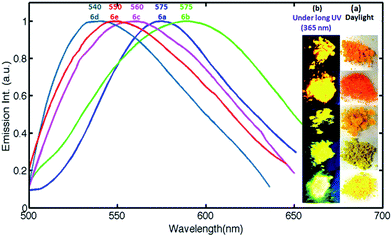 | ||
| Fig. 3 Normalized solid state fluorescence plots for dyes 6a–6e. Inset showing photograph of solid dyes under UV light and daylight. | ||
Theoretical results
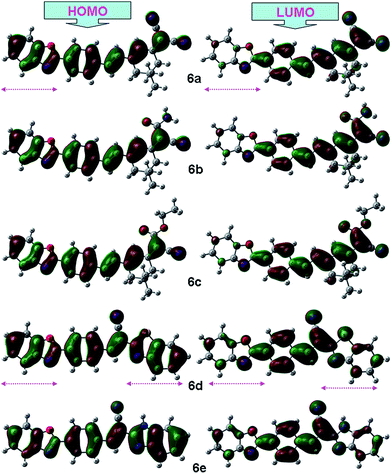
The ground state geometry of the compounds 6a–6e was optimized in vacuum using Density Functional Theory (DFT). The functional used was B3LYP which combines Becke's three parameter exchange functional (B3) with the nonlocal correlation functional by Lee, Yang, and Parr (LYP). The basis set used for all atoms was 6-31G(d,p). Fig. 4 gives clear elucidation of electron density distribution on HOMO and LUMO. HOMO of the dyes is mainly located on the benzoxazole derivative while the LUMO of the dyes are contributed by strong electron withdrawing cyano group. This clearly denotes an efficient charge migration from the donor unit to the acceptor segment on HOMO to LUMO electronic excitation. Variation in dipole moment explains the electron withdrawing capabilities of functional group attached to benzoxazole moieties. The vertical excitation energies at the ground state equilibrium geometries were calculated with TD-DFT. Table 4 signifies the close agreement between observed and theoretical values of HOMO–LUMO and band gap. It also represents theoretical vertical excitation values determined by using TD-B3LYP/6.31G(d,p) in gas phase.| Dyes | ELUMO; EHOMO | ΔEa (eV) | Dipole moment (Debye) | λabs, nm | Excitation energy (eV) | λem, nm |
|---|---|---|---|---|---|---|
| a ΔE = ELUMO – EHOMO.b Oscillatory strength (f). | ||||||
| 6a | −0.11255; −0.21839 | 0.10584 | 7.98 | 449 (1.54)b | 2.7645 | 495 (1.70)b |
| 365 (0.42) | 3.3969 | |||||
| 6b | −0.10014; −0.20918 | 0.10904 | 2.67 | 435 (1.74) | 1.7361 | 487 (1.90) |
| 357 (0.27) | 0.2697 | |||||
| 6c | −0.10264; −0.21073 | 0.10809 | 4.32 | 440 (1.73) | 2.8185 | 492 (1.90) |
| 360 (0.27) | 3.4408 | |||||
| 6d | −0.10404; −0.21995 | 0.11591 | 2.26 | 423 (1.53) | 2.9347 | 485 (1.58) |
| 377 (0.03) | 3.2875 | |||||
| 6e | −0.09995; −0.21296 | 0.11301 | 0.93 | 434 (1.31) | 2.8541 | 508 (1.08) |
| 390 (0.14) | 3.1739 | |||||
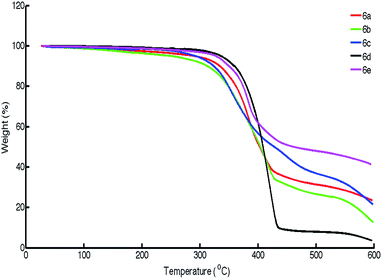
Thermal and photochemical stability study
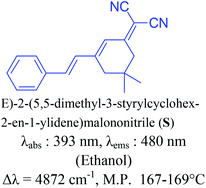
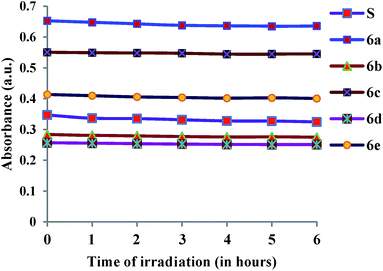
The thermal degradation temperatures (Td) of dyes 6a–6e were recorded by thermogravimetric analysis (Fig. 5). The dyes were heated at the rate of 10 °C min−1 from 30–600 °C under inert atmosphere of nitrogen gas. These novel colorants showed excellent thermal stability. The comparisons of the Td (decomposition temperature) at 95% weight of the sample, show that the thermal stability of dyes decreases in the order 6d (336 °C) > 6e (327 °C) > 6c (293 °C) > 6a (291 °C) > 6b (252 °C).Photochemical stability was next investigated by irradiating the solution of dyes, in acetonitrile at room temperature in UV cabinets (at 254 nm, 0.15 mW cm−2) for a period of 6 h.26 In the photostability study, we have tried to discuss about the effect of UV irradiation on photostability of the dye molecules in terms of the absorption. No changes in absorbance intensity of the dyes were observed on plotting the absorbance versus duration of irradiation, which inferred that the dyes are extremely photo-chemically stable (Fig. 6 and S6†). The photochemical stability was compared by carrying out a control experiment with similar molecule (S). It was observed that the absorbance intensity of S was comparable with synthesized molecule 6a (Fig. 6).
Conclusions
Novel dyes synthesized exhibited good thermal stability and solid state luminescence property. Their photophysical properties reveal that they can be potential candidates for OLED, NLO and polymeric security application. Emission spectra of these dyes were red shifted in solvents of varying polarity showing positive solvatofluorism. Comparison with reported analogues showed the advantage of synthesized dye in terms of photophysical and thermal properties.Acknowledgements
Authors are thankful to UGC-CAS for providing financial assistance and to the Institute of Chemical Technology (ICT), SAIF IIT-Bombay for recording FT-IR, HR-MS and TIFR Mumbai for 1H and 13C-NMR.Notes and references
- P. Peumans, V. Bulović and S. R. Forrest, Appl. Phys. Lett., 2000, 76, 3855–3857 CrossRef CAS
.
- B. Liu, W. Zhu, Q. Zhang, W. Wu, M. Xu, Z. Ning, Y. Xie and H. Tian, Chem. Commun., 2009, 1766–1768 RSC
.
- M. A. Haidekker, T. P. Brady, D. Lichlyter and E. A. Theodorakis, Bioorg. Chem., 2005, 33, 415–425 CrossRef CAS PubMed
.
- G.-S. Jiao, L. H. Thoresen and K. Burgess, J. Am. Chem. Soc., 2003, 125, 14668–14669 CrossRef CAS PubMed
.
- P. E. Burrows, Z. Shen, V. Bulovic, D. M. McCarty, S. R. Forrest, J. A. Cronin and M. E. Thompson, J. Appl. Phys., 1996, 79, 7991–8006 CrossRef CAS
.
- S. Speiser and N. Shakkour, Appl. Phys. B, 1985, 38, 191–197 CrossRef
.
- E.-M. Lee, S.-Y. Gwon, B.-C. Ji, J.-S. Bae and S.-H. Kim, Fibers Polym., 2011, 12, 288–290 CrossRef CAS
.
- V. K. Ol'khovik and A. G. Baran, Russ. J. Org. Chem., 2010, 46, 1185–1191 CrossRef
.
- M. M. M. Raposo, S. P. G. Costa and H. Nmr, Adv. Colour Sci. Technol., 2002, 5, 3–7 Search PubMed
.
- S.-H. Kim, J.-Z. Cui, J.-Y. Park, E.-M. Han and S.-M. Park, Dyes Pigm., 2003, 59, 245–250 CrossRef CAS
.
- M. Mac, T. Uchacz, A. Danel, M. A. Miranda, C. Paris and U. Pischel, Photochem. Photobiol. Sci., 2008, 7, 633–641 CAS
.
- J. Massue, D. Frath, G. Ulrich, P. Retailleau and R. Ziessel, Org. Lett., 2012, 14, 230–233 CrossRef CAS PubMed
.
- J. Kabatc, B. Jedrzejewska, A. Bajorek and J. Paczkowski, J. Fluoresc., 2006, 16, 525–534 CrossRef CAS PubMed
.
- K. Benelhadj, W. Muzuzu, J. Massue, P. Retailleau, A. Charaf-Eddin, A. D. Laurent, D. Jacquemin, G. Ulrich and R. Ziessel, Chem.–Eur. J., 2014, 20, 12843–12857 CrossRef CAS PubMed
.
- R. F. Affeldt, A. C. de Amorim Borges, D. Russowsky and F. Severo Rodembusch, New J. Chem., 2014, 38, 4607–4614 RSC
.
- Z. Yuan, Q. Tang, K. Sreenath, J. T. Simmons, A. H. Younes, D. Jiang and L. Zhu, Photochem. Photobiol., 2015, 91, 586–598 CrossRef CAS PubMed
.
- Y. Xu, L. Xiao, S. Sun, Z. Pei, Y. Pei and Y. Pang, Chem. Commun., 2014, 50, 7514–7516 RSC
.
- A. Reiser, L. J. Leyshon, D. Saunders, M. V. Mijovic, A. Bright and J. Bogie, J. Am. Chem. Soc., 1972, 94, 2414–2421 CrossRef CAS
.
- B. S. Singh, H. R. Lobo, G. Krishna Podagatlapalli, S. Venugopal Rao and G. S. Shankarling, Opt. Mater., 2013, 35, 962–967 CrossRef CAS
.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS
.
- M. Shaikh, J. Mohanty, P. K. Singh, A. C. Bhasikuttan, R. N. Rajule, V. S. Satam, S. R. Bendre, V. R. Kanetkar and H. Pal, J. Phys. Chem. A, 2010, 114, 4507–4519 CrossRef CAS PubMed
.
- X. Zhang and Y. Chen, Dyes Pigm., 2013, 99, 531–536 CrossRef CAS
.
- R. Lemke, Chem. Ber., 1970, 103, 1894–1899 CrossRef CAS
.
- T. M. Kolev, D. Y. Yancheva and B. A. Stamboliyska, Spectrochim. Acta, Part A, 2003, 59, 3325–3335 CrossRef
.
- T. A. Fayed, S. E.-D. H. Etaiw and H. M. Khatab, J. Photochem. Photobiol., A, 2005, 170, 97–103 CrossRef CAS
.
- S. R. Menon and G. S. Shankarling, Color. Technol., 2011, 127, 383–389 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic data, HRMS, 1H and 13C NMR. See DOI: 10.1039/c4ra12908c |
| This journal is © The Royal Society of Chemistry 2015 |