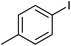
Palladium on manganese ferrite: an efficient catalyst for one pot synthesis of primary amides from iodobenzene†
Vilas Gangadhar Jadhav,
Jeevan Manohar Bhojane and
Jayashree Milind Nagarkar*
Department of Chemistry, Institute of Chemical Technology, Matunga, Mumbai, 400019, India. E-mail: jm.nagarkar@ictmumbai.edu.in; Fax: +91 22 33611020; Tel: +91 22 33611111/2222
First published on 12th December 2014
Abstract
Amidation of aryl iodide in one pot is reported using Pd–MnFe2O4 as a catalyst. The catalyst was characterized by various techniques such as XRD, FEG-SEM, EDS, TEM, BET surface area and ICP AES. K4[Fe(CN)6]is used as a non toxic cyanation reagent for in situ generation of benzonitrile and hydrolyzed as soon as it formed. The catalyst was found to be efficient and can be used for several cycles without loss in activity. Good to excellent yields of primary amides were obtained.
1. Introduction
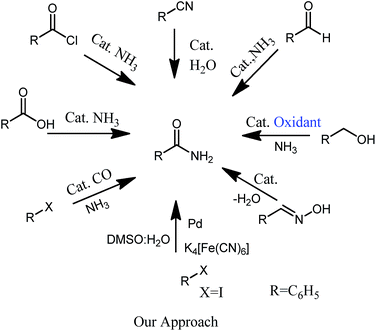
Primary amides are the most important functionality in pharmaceuticals, natural products, agrochemicals and biologically active molecules.1–3 Generally, primary amides are synthesized either by the reaction of carboxylic acid or its derivatives with ammonia or its equivalent.4 They are also synthesized by different methodologies such as catalytic rearrangement of aldoximes,6 reaction of aldehydes with ammonia,7 oxidation of benzylic alcohols in the presence of ammonia8 and catalytic oxidation of benzyl amines.9 Aminocarbonylation of aryl halide by using carbon monoxide and ammonia or ammonia equivalent is also reported as the efficient method for the synthesis of primary amides. However, this process involves handling of toxic gas and reduces its use at kilogram level.5 Aryl halides with ammonia in presence of metal carbonyls also have been used for the synthesis of primary amides, but it leads to release of toxic carbon monoxide gas and also the metal carbonyl complexes are volatile and unstable.10 Hydration of nitriles is an atom economical process and numbers of catalytic methods have been developed for nitrile synthesis.11 Hence preparation of nitriles as an intermediate for amide synthesis is of great importance. Nitriles are prepared by reaction of aryl halides with various nitrile sources such as alkali cyanide, CuCN, TMSCN and K4[Fe(CN)6] salt in presence of transition metal catalyst. Among these K4[Fe(CN)6] is used as a less toxic and cheaper cyanide source for cyanation of aryl halides.12Recently oxidation of benzyl amine to primary amide by using OMS-2 catalyst was reported.9c However, the reaction requires very high pressure (6 bar) and temperature (160 °C) and also gives side products, where as we have reported general synthesis of primary amides at mild reaction conditions using magnetically retrievable catalyst.
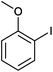
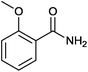
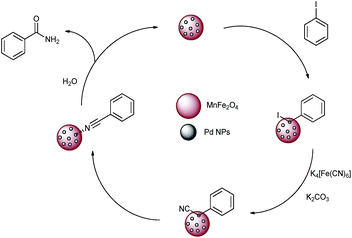
Magnetically separable catalyst has attracted much attention in the area of heterogeneous catalyst as it is easily separated by external magnet and can be used several times without losing its activity.13 Super paramagnetic materials, spinel ferrites MFe2O4 (M = Ni, Co, Mn, Zn) are one of the important advance materials used in drug delivery and biomedical applications such as MRI.14 Besides this, they are also found to be good support for transition metal catalysts.15 In particular, palladium supported on super paramagnetic material is the most important heterogeneous catalyst as it offers high surface area and easy separation. In continuation of our work with metal nano particles supported on super paramagnetic material,15b herein we report palladium nanoparticles (PdNPs) supported on MnFe2O4 and its application in one pot synthesis of primary amides (Fig. 1).
Manganese ferrite (MnFe2O4) nano crystals possess higher magnetization as compared to magnetite, CoFe2O4 and NiFe2O4 nanoparticles.16 Here we have carried out the tandem synthesis of primary amide from iodobenzene via in situ generation of benzonitrile in presence of DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solvent system using Pd nano particles supported on MnFe2O4. This is the first report of the application of the heterogeneous catalyst for the synthesis of primary amide and that too using nontoxic nitrile source K4[Fe(CN)6]. Very good yields of the desired products were obtained.
H2O solvent system using Pd nano particles supported on MnFe2O4. This is the first report of the application of the heterogeneous catalyst for the synthesis of primary amide and that too using nontoxic nitrile source K4[Fe(CN)6]. Very good yields of the desired products were obtained.
2. Experimental
2.1 Catalyst preparation
Catalyst was prepared by using ultrasound assisted coprecipitation method. FeCl3·6H2O (9.5 mmol, 50 mL) and MnCl2·4H2O (4.4 mmol, 50 mL) solutions were added in 250 mL round bottom flask followed by addition of 0.5 g KCl and PdCl2 (60 mg). This mixture was placed in ultrasound bath for 5 min. Then pH of this mixture was adjusted at 12 by adding 3 N NaOH followed by heating at 60 °C for 30 min. The obtained catalyst was filtered and washed with distilled water several times and then by ethanol to remove water. The catalyst was dried under vacuum at 60 °C for 24 h, and subsequently at 200 °C for 4 h.2.2 Characterization of material
The as synthesized catalyst was characterized by various techniques such as X-ray diffraction (XRD), Field emission gun scanning electron microscopy (FEG-SEM), Transmission electron microscopy (TEM), FTIR, ICP-AES analysis, BET surface area. The XRD analysis was performed on Shimadzu XRD 2400 instrument using Cu Kα radiation (λ = 1.5406 Å) with scanning rate 2 degree per minute. TEM analysis was performed over PHILIPS 2200 instrument. FEG-SEM analysis was performed on TESCAN MIRA Instrument. The energy dispersive X-ray spectral analysis (EDS) image was recorded with an Oxford instrument at 10 kV. Beam intensity was kept high to get good response by the detector. Inductively coupled plasma atomic absorption spectrometry (ICP AES) analysis was performed on ARCOS from M/s. Spectro, Germany. GC analysis is performed over PerkinElmer Clarus 480 instrument. GC-MS Spectra recorded over Shimadzu QP-2010 Instrument. 1H and 13C NMR spectra's were recorded on Agilent 400 MHz and 100 MHz instrument respectively.3. Results and discussion
XRD analysis showed peak at 2θ = 30.1, 35.14, 43.41, 54.601, 57.0671 and 63.6651 which represent the Bragg reflections from the (220), (311), (400), (422), (511) and (440) planes respectively. The crystalline data matches with the JCPDS Card no. (74-2403). Palladium peaks at 40, 46 θ values were not observed because of the low loading of the palladium (Fig. 2a). Displacing value from XRD and TEM SEAD pattern is same and found to be 2 Å (Fig. 3c and d). | ||
| Fig. 3 FEG-SEM image (a) fresh and (b) reused catalyst after fifth cycle. (c) TEM image of catalyst and (d) SEAD pattern. | ||
The elemental analysis of Pd–MnFe2O4 was confirmed by EDS analysis as shown in Fig. 2b. The distribution of elements in Pd–MnFe2O4 was Mn = 11.45%, Fe = 28.15%, O = 59.31% and Pd = 1.09%.
FEG-SEM analysis shows highly crystalline morphology of the catalyst (Fig. 3a and b).
The TEM image of Pd–MnFe2O4 NPs (Fig. 3c) clearly indicates that NPs are uniform in size with average particle size less than 45 nm. This is in accordance with the average crystallite size obtained by using the Debye–Scherrer equation. The image also exhibits that the Pd NPs on MnFe2O4 are well dispersed. Pd NPs size ranges from 2–10 nm.
From the N2 adsorption–desorption isotherm of Pd–MnFe2O4 NPs, the BET surface area of the particles was found to be 24.9346 m2 g−1 and the adsorption average pore width was 315 Å. Single point surface area at P/Po = 0.254238207![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.5190 m2 g−1 (Fig. 2 ESI†).
24.5190 m2 g−1 (Fig. 2 ESI†).
Reaction of iodobenzene with K4[Fe(CN)6] was treated as a model reaction and various reaction parameters were optimized for this reaction. Initially (1 mmol) of iodobenzene was treated with 0.25 mmol of K4[Fe(CN)6] in the presence of Pd–MnFe2O4 catalyst (30 mg, 1 mol% Pd), K2CO3 as a base in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system, at 100 °C, which offered 58% yield of benzamide (Table 1, entry 1). Series of experiments were performed to optimize the various reaction parameters such as catalyst loading, solvent, base and temperature of the reaction (Scheme 1).
1) solvent system, at 100 °C, which offered 58% yield of benzamide (Table 1, entry 1). Series of experiments were performed to optimize the various reaction parameters such as catalyst loading, solvent, base and temperature of the reaction (Scheme 1).
| Entry | Pd–MnFe2O4 (mol%) | Base | Solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl halide (1 mmol), K4[Fe(CN)]6 (0.25 mmol), time 12 h, base (1.2 mmol).b Isolated yield.c Time 18 h.d 24 h.e Benzonitrile 92%. | |||||
| 1 | 0.5 | K2CO3 | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 58 |
| 2 | 0.5 | K2CO3 | NMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 64 |
| 3 | 0.5 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 70 |
| 4 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 78 |
| 5 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 84 |
| 6 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 88 |
| 7 | 2 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 88 |
| 8 | 3 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 88 |
| 9 | 1 | Cs2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 74 |
| 10 | 1 | TEA | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | Trace |
| 11 | 1 | DBU | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | Trace |
| 12 | 1 | KOH | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 20 |
| 13 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
90 | 19 |
| 14 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
120 | 91 |
| 15 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
140 | 90 |
| 16 | 1 | K2CO3 | H2O | 110 | — |
| 17 | 1 | K2CO3 | DMSO | 110 | —e |
| 18 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 90c |
| 19 | 1 | K2CO3 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 90d |
Loading of the catalyst was also the important parameter and it was found that 1 mmol of the catalyst was enough to achieve the maximum yield of the desired product (Table 1, entry 4). Solvent study was the important part for this transformation. Various solvent systems such as DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, DMSO
H2O, DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and NMP
H2O and NMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O were used out of which DMSO
H2O were used out of which DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be the most suitable solvent system for this transformation (Table 1, entry 5). When reaction was carried out in DMSO solvent only, benzonitrile was formed (Table 1, entry 17). Iodobenzene was in situ converted to benzonitrile by the cyanating reagent K4[Fe(CN)6] which immediately gets hydrated to benzamide in the presence of water.
1) was found to be the most suitable solvent system for this transformation (Table 1, entry 5). When reaction was carried out in DMSO solvent only, benzonitrile was formed (Table 1, entry 17). Iodobenzene was in situ converted to benzonitrile by the cyanating reagent K4[Fe(CN)6] which immediately gets hydrated to benzamide in the presence of water.
The reaction was also carried out with various inorganic and organic bases such as TEA, DBU, K2CO3, KOH and Cs2CO3, out of which K2CO3 was found to be the most efficient base giving maximum yield of the benzamide (Table 1, entry 8). The reaction was also carried out at various temperatures under optimized reaction conditions. The product yield increased dramatically from 19% to 90% when temperature increased from 90 °C to 110 °C whereas beyond 110 °C no significant increase in the product yield with increase in temperature was observed (Table 1, entries 13–15).
We also studied the effect of concentration of K4[Fe(CN)6] salt on the product yield by increasing the amount from 0.1 mmol to 0.25 mmol. Conversion of aryl halide to benzonitrile was 100% at 0.25 mmol concentration of K4[Fe(CN)6]. The reaction was also carried out at various time intervals and 100% conversion was obtained in 18 h. For optimized reaction TON and TOF‡ for amidation of iodobenzene was found to be 90 and 5 h−1.
The applicability of the protocol was examined for various substituted aryl iodides (Table 2). It was found that electron rich substrate gave very good yield of the desired product.
In case of p-NO2 iodobenzene electron withdrawing effect was more prominent giving moderate yield of the desired products. However, in m-nitro iodobenzene the yield was increased due to minimum electron withdrawing effect. Results are summarized in Table 2 (entries 6 and 11).
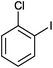
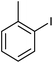
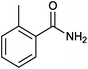
Donating substituents at ortho positions were well tolerated giving good yield of the corresponding primary amides. We further studied amidation of bromobenzene as well as chlorobenzene and observed that bromobenzene gave only 20% yield of the desired product whereas chlorobenzene was unreactive with optimized reaction conditions (Table 2, entries 2 and 3). Heteroaryl iodide also gave good yield of corresponding amide (Table 2, entry 16).
3.1 Heterogeneous test for the catalyst
We also examined the heterogeneous nature of the catalyst by hot filtration test. The catalyst was separated by using external magnet from reaction mass after half of the reaction time and the filtrate was stirred for required time under same reaction conditions. The reaction did not proceed further, which indicated that there is no leaching of palladium during the reaction. We also carried out the ICP AES analysis to test the recyclability of the catalyst for first and fifth cycles which showed that the palladium leaching was below detectable level (0.01 ppm) (Fig. 4 and 6).Plausible mechanism suggests that iodobenzene is oxidatively added to the catalyst and then in presence of K4[Fe(CN)6] salt gives benzonitrile which subsequently get hydrolyzed in presence of water and catalyst to give primary amide (Fig. 5).
4. Conclusions
In summary, we have developed the simple and practically applicable protocol for the synthesis of primary amides from aryl iodides. Pd–MnFe2O4 is the first heterogeneous catalyst for such transformation. Catalyst was found to be efficient for the transformation of iodobenzenes to respective amides. K4[Fe(CN)6] was used as the non toxic cyanating reagent and in situ generated benzonitrile was hydrated as soon as it forms. The catalyst can be retrieved using external magnet and reused up to five consecutive cycles without much loss in its activity.Acknowledgements
The authors are very thankful to the UGC-SAP, New Delhi, India for awarding the fellowship.Notes and references
- G. Arthur. The Amide Linkage: Selected Structural Aspects in Chemistry, Biochemistry, and Materials Science, Wiley-Interscience, 2000 Search PubMed.
- S. Budavari, The Merck Index, Merck, Rahway, USA, 11th edn, 1989 Search PubMed.
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471 CrossRef CAS PubMed.
- (a) G. G. Arzoumanidis and F. C. Rauch, J. Org. Chem., 1981, 46, 3930–3932 CrossRef CAS; (b) C. L. Allen and J. M. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC; (c) E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC; (d) H. Lundberg, F. Tinnis, N. Selander and H. Adolfsson, Chem. Soc. Rev., 2014, 43, 2714 RSC.
- (a) P. G. Alsabeh, M. Stradiotto, H. Neumann and M. Beller, Adv. Synth. Catal., 2012, 354, 3065–3070 CrossRef CAS; (b) X.-F. Wu, H. Neumann and M. Beller, Chem.–Asian J., 2010, 5, 2168–2172 CrossRef CAS PubMed; (c) X.-F. Wu, H. Neumann and M. Beller, Chem.–Eur. J., 2010, 16, 9750–9753 CrossRef CAS PubMed; (d) X.-F. Wu, H. Neumann and M. Beller, Chem.–Eur. J., 2012, 18, 419–422 CrossRef CAS PubMed; (e) X.-F. Wu, J. Schranck, H. Neumann and M. Beller, ChemCatChem, 2012, 4, 69–71 CrossRef CAS; (f) T. Xu and H. Alper, Tetrahedron Lett., 2013, 54, 5496–5499 CrossRef CAS PubMed; (g) S. T. Gadge and B. M. Bhanage, Synlett, 2014, 25, 85–88 CAS.
- (a) A. Martinez-Asencio, M. Yus and D. J. Ramon, Tetrahedron, 2012, 68, 3948–3951 CrossRef CAS PubMed; (b) F. Li, P. Qu, J. Ma, X. Zou and C. Sun, ChemCatChem, 2013, 5, 2178–2182 CrossRef CAS; (c) N. A. Owston, A. J. Parker and J. M. Williams, Org. Lett., 2007, 9, 3599–3601 CrossRef CAS PubMed; (d) C. Sun, P. Qu and F. Li, Catal. Sci. Technol., 2014, 4, 988–996 RSC.
- (a) U. B. Patil, A. S. Singh and J. M. Nagarkar, RSC Adv., 2014, 4, 1102–1106 RSC; (b) M. A. Ali and T. Punniyamurthy, Adv. Synth. Catal., 2010, 352, 288–292 CrossRef CAS.
- (a) K. Yamaguchi, H. Kobayashi, T. Oishi and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 544–547 CrossRef CAS PubMed; (b) K. Yamaguchi, H. Kobayashi, Y. Wang, T. Oishi, Y. Ogasawara and N. Mizuno, Catal. Sci. Technol., 2013, 3, 318–327 RSC; (c) R. Nie, J. Shi, S. Xia, L. Shen, P. Chen, Z. Hou and F. Xiao, J. Mater. Chem., 2012, 22, 18115–18115 RSC; (d) R. Ohmura, M. Takahata and H. Togo, Tetrahedron Lett., 2010, 51, 4378–4381 CrossRef CAS PubMed; (e) R. Das and D. Chakraborty, Catal. Commun., 2012, 26, 48–53 CrossRef CAS PubMed; (f) X.-Q. Li, W.-K. Wang, Y.-X. Han and C. Zhang, Adv. Synth. Catal., 2010, 352, 2588–2598 CrossRef CAS.
- (a) X.-F. Wu, C. B. Bheeter, H. Neumann, P. H. Dixneuf and M. Beller, Chem. Commun., 2012, 48, 12237–12239 RSC; (b) J. W. Kim, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2008, 47, 9249–9251 CrossRef CAS PubMed; (c) Y. Wang, H. Kobayashi, K. Yamaguchi and N. Mizuno, Chem. Commun., 2012, 48, 2642–2644 RSC.
- (a) W. Ren and M. Yamane, J. Org. Chem., 2010, 75, 8410–8415 CrossRef CAS PubMed; (b) W. Ren and M. Yamane, J. Org. Chem., 2010, 75, 3017–3020 CrossRef CAS PubMed.
- (a) J. Mauger, T. Nagasawa and H. Yamada, Tetrahedron, 1989, 45, 1347–1354 CrossRef CAS; (b) A. Goto, K. Endo and S. Saito, Angew. Chem., Int. Ed., 2008, 47, 3607–3609 CrossRef CAS PubMed; (c) R. S. Ramon, N. Marion and S. P. Nolan, Chem.–Eur. J., 2009, 15, 8695–8697 CrossRef CAS PubMed; (d) M. Muranaka, I. Hyodo, W. Okumura and T. Oshiki, Catal. Today, 2011, 164, 552–555 CrossRef CAS PubMed; (e) E. Bolyog-Nagy, A. Udvardy, F. Joo and A. Katho, Tetrahedron Lett., 2014, 55, 3615–3617 CrossRef CAS PubMed; (f) S. Kumar and P. Das, New J. Chem., 2013, 37, 2987–2990 RSC; (g) V. Polshettiwar and R. S. Varma, Chem.–Eur. J., 2009, 15, 1582–1586 CrossRef CAS PubMed; (h) T. Hirano, K. Uehara, K. Kamata and N. Mizuno, J. Am. Chem. Soc., 2012, 134, 6425–6433 CrossRef CAS PubMed; (i) Nippon Shokubai Co. Ltd, Patent: JP2005/170821 A, 2005; (j) Y. Wang, F. Wang, Q. Song, Q. Xin, S. Xu and J. Xu, J. Am. Chem. Soc., 2013, 135, 1506–1515 CrossRef CAS PubMed; (k) K.-T. Liu, M.-H. Shih, H.-W. Huang and C.-J. Hu, Synthesis, 1988, 9, 715–717 CrossRef.
- (a) T. Schareina, A. Zapf and M. Beller, Chem. Commun., 2004, 1388 RSC; (b) T. Schareina, R. Jackstell, T. Schulz, A. Zapf, A. Cotte, M. Gotta and M. Beller, Adv. Synth. Catal., 2009, 351, 643 CrossRef CAS; (c) J. Huiliang, J. Jianzhong, W. Huixian and C. Chun, Catal. Lett., 2013, 143, 1195–1199 CrossRef PubMed.
- (a) W. L. Choon and I. S. Lee, Nano Today, 2010, 5, 412–434 CrossRef PubMed; (b) V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed; (c) A. H. Latham and M. E. Williams, Acc. Chem. Res., 2008, 41, 411–420 CrossRef CAS PubMed; (d) S. A. Sarode, J. M. Bhojane and J. M. Nagarkar, Tetrahedron Lett., 2015, 56, 206–210 CrossRef PubMed; (e) R. S. Shelkar, S. H. Gund and J. M. Nagarkar, RSC Adv., 2014, 4, 53387–53396 RSC.
- J. Lu, S. Ma, J. Sun, C. Xia, C. Liu, Z. Wang, X. Zhao, F. Gao, Q. Gong, B. Song, X. Shuai, H. Ai and Z. Gua, Biomaterials, 2009, 30, 2919–2928 CrossRef CAS PubMed.
- (a) K. K. Senapati, S. Roy, C. Borgohain and P. Phukan, J. Mol. Catal. A: Chem., 2012, 352, 128–134 CrossRef CAS PubMed; (b) A. S. Singh, U. B. Patil and J. M. Nagarkar, Catal. Commun., 2013, 35, 11–16 CrossRef CAS PubMed; (c) S. R. Borhade and S. B. Waghmode, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2008, 47, 1549–1554 Search PubMed; (d) D. Wang and D. Astruc, Molecules, 2014, 19, 4635–4653 CrossRef CAS PubMed; (e) N. Baig and R. S. Verma, Chem. Commun., 2013, 49, 752–770 RSC; (f) V. Polshettiwar and R. S. Verma, Org. Biomol. Chem., 2009, 7, 37–40 RSC.
- (a) U. I. Tromsdorf, N. C. Bigall, M. G. Kaul, O. T. Bruns, M. S. Nikolic and B. Mollwitz, Nano Lett., 2007, 7, 2422 CrossRef CAS PubMed; (b) J. H. Lee, Y. M. Huh, Y. Jun, J. Seo, J. Jang and H. T. Song, Nat. Med., 2007, 13, 95 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization of the catalyst, mass and 1H NMR spectrum of representative compounds are given. See DOI: 10.1039/c4ra12827c |
| ‡ TOF = [mole of product/(mole catalyst × h)]. |
| This journal is © The Royal Society of Chemistry 2015 |