Insight into two antioxidants binding to the catalase NADPH binding site from traditional Chinese medicines
Abstract
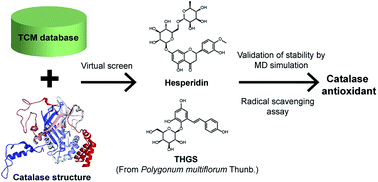
Catalase is an important enzyme, which performs the decomposition of two molecules of hydrogen peroxide into water molecules and oxygen, in an aerobic organism. Deficient or inactive catalase are implicated in cell damage and lead to inflammation, aging and cancer. In order to develop a novel natural product that prevents inactive catalase generation, the world largest traditional Chinese medicine (TCM) database (http://tcm.cmu.edu.tw/) was employed for this study, which combined high-throughput virtual screening and molecular dynamics (MD) simulation to investigate potent natural compounds for keeping catalase active. We found two natural products, hesperidin and 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (THSG), and they present high binding affinity with catalase. The results of MD simulation show that THSG is the most stable in trajectory analysis in all simulations. Moreover, THSG can make the catalase structure more compact during the process of MD simulation. In addition, the radical scavenging assay showed that THSG has more potential antioxidant activity than hesperidin. Therefore, we regard if the natural TCM compound, THSG, could be used to develop potential drugs that might have similar effect to keep catalase active and prevent the inactive form generation by hydrogen peroxide.

 Please wait while we load your content...
Please wait while we load your content...