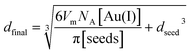Gold nucleation inhibition by halide ions: a basis for a seed-mediated approach†
R.
Moiraghi‡
*,
O. A.
Douglas-Gallardo
,
E. A.
Coronado
,
V. A.
Macagno
and
M. A.
Pérez
INFIQC, Departamento de Fisicoquímica, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Córdoba, Argentina. E-mail: moiraghi@ifir-conicet.gov.ar
First published on 29th January 2015
Abstract
In the present work, we examine the effect of halide ions on gold nucleation, a typical synthetic variable in the wet-chemical production of gold nanostructures. It was found that the homogeneous nucleation of gold by the chemical reduction of aqueous gold ions is kinetically quenched by an increase in the concentration of halide ions, and this effect grows stronger as the Au–halide complex stability increases. The nucleation quenching is not exclusively related to a specific reducing agent, but appears to be a more general behavior, and is affected by the pH of the media. While no nucleation is observed, Au(I) metastable species coexist together with the reducer, constituting metastable solutions. It is demonstrated that nucleation inhibition by halide ions can be employed as a basis for a seed-mediated approach to produce gold nanostructures. The metastable solutions are proved to function as growth baths, where Au(I) reduction is triggered on the surface of previously synthesized gold nanoparticles, driving their growth in the absence of secondary nucleation. It is also shown how, with this approach, the synthesis conditions can be rationally designed to obtain gold nanoparticles with the desired properties in a controlled and reproducible fashion.
Introduction
The synthesis of noble metal nanoparticles (NPs) is an active research area; thousands of new synthetic protocols are reported every year. There have been great advances in producing metal NPs with several morphological and compositional features with novel properties and applications.1–4 Nevertheless, the physicochemical processes and the mechanisms involved in NPs formation are still scarcely known. Currently, two main processes in NPs formation can be differentiated: nucleation and growth. In the LaMer's mechanism, proposed for sulphur sols formation,5 self-nucleation (homogeneous nucleation) is claimed to be very fast, and the growth is claimed to be diffusion-controlled. Beyond this often quoted mechanism, in metallic systems, nucleation has been shown to be continuous,6–13 and growth has been shown to be autocatalytic6–13 and aggregative.14 Furthermore, other processes have been identified such as Ostwald ripening,15,16 digestive ripening17 and oxidative etching.18 All these factors may contribute to the size, shape and stability of the NPs. In a typical synthesis, nevertheless, because many of these processes take place simultaneously, it is difficult to unravel the influence of each process in the final NPs properties. Likewise, it is not clear how the diverse synthesis variables contribute to each of these processes. To plan a synthesis, therefore, it is consensual that one of the keys in controlling the size dispersion is to “separate” the nucleation and growth stages in time.19 The search for synthesis conditions to accomplish this separation is not trivial; in addition, other requirements may have to be considered, e.g., suspension media, long-term stability, and functionalization. In the synthesis of very small NPs, for example, the use of strong reducing agents, such as borohydride ion, and the use of stabilizing agents that are tightly bound to the NPs, such as thiols on gold surfaces, has been proved to be effective to favour nucleation over growth.20 Nevertheless, for many applications, such as catalysis or sensing, the presence of strongly bound stabilizing agents represents a serious disadvantage. Another example is the hot-injection method, where the separation of nucleation and growth has been successfully achieved by applying temperature ramps.21 Much more extensively employed, seed-mediated approaches have accomplished this separation using different chemical environments.22–24 In particular, the quaternary ammonium-based seed-mediated growth method first reported by Murphy's group25–27 has been successfully used to synthesize anisotropic gold NPs (AuNPs) of various shapes, such as nanorods,26–28 nanocubes,29 nanostars,29 bypiramids,30 nanoprisms,31 dumbbell-like,32 and jack-shaped.33 This synthesis involves seeding small AuNPs (seeds) into reactive solutions, where AuNPs grow in the absence of homogeneous nucleation. These reactive solutions, usually called growth baths, contain the Au(III) precursor, a quaternary ammonium salt, typically hexadecyltrimethylammonium bromide (CTAB), and ascorbic acid (AA) as the reducing agent. In this case, the absence of homogeneous nucleation during the growth of the AuNPs is attributed to the weak nature of the reducing agent employed (ascorbic acid).34 In this synthesis, as in many other synthetic procedures to obtain gold nanostructures, halide ions are present. Generally, investigations about the effect of halide ions are mainly focused for determining their role in the anisotropic growth.35–37In the present work, we study the chemistry of aqueous halide–gold systems and the consequences of this chemistry for gold nucleation by the chemical reduction of gold ions. The effects of experimental variables, such as the nature of the reducing agent and the pH of the media, are explored. Owing to the rich spectroscopic features that Au(III) and Au(0) species display, the studies were carried out mainly by employing UV-Vis spectroscopy. The implications of the present investigations for the quaternary ammonium-based seed-mediated growth method are discussed. In addition, it is shown how the knowledge developed in this work constitutes a basis for a seed-mediated approach for gold nanostructures. It is demonstrated that many seed-mediated methods can be devised using the basic recipe of Au(III) precursor, halide ions and a mild reducing agent by varying, for example, the nature of the reducing agent or seed, or by adding other co-reactants such as polymers or amphiphilic molecules.
Results and discussion
Halide ions effect
When bromide ions are added to a solution of HAuCl4, an increase in the intensity of yellow colour is observed. This phenomenon is the result of the exchange of the hydroxyl and chloride ions by bromide ions in the coordination sphere of Au(III). The spectral sequence for increasing concentrations of bromide ions is displayed in Fig. 1a. As the bromide ion concentration increases, the absorption increases in the 350–650 nm region because the ligand exchange process takes place.38 The absorption growth above 400 nm is responsible for the observed increase in the intensity of yellow colour. No major differences are noticed in the spectroscopic behaviour for bromide ion concentrations higher than 5 mM (the spectra are overlapped), indicating that the ligand exchange reaction to the formation of AuBr4− is complete. This complex exhibits a strong absorption peak at 382 nm, followed by a shoulder and a tail that quickly decreases up to 600 nm to reach very small values along the rest of the spectral range. When hydroquinone (H2Q) is added to these solutions, drastic spectroscopic changes are observed (Fig. 1b). The absence of the characteristic spectral features of Au(III) complexes for all concentrations indicates that the reduction reaction took place. However, the resulting spectral profiles show a strong dependence on the bromide ion content. At low bromide ion concentrations (Fig. 1 black and red lines), the reactive solutions show extinction peaks between 700 nm and 800 nm. These features are associated with the surface plasmon resonance (SPR) of large AuNPs (diameter ∼ 150 nm)39 produced by the reduction of Au(III), according to reactions (1), (2) and/or (3):| AuL4(ac)− + H2Q(ac) → AuL2(ac)− + Q + 2H(ac)+ + 2L2(ac)− | (1) |
| AuL2(ac)− + ½H2Q(ac) → Au(NP) + ½Q + H(ac)+ + 2L2(ac)− | (2) |
| 3AuL2(ac)− → AuL4(ac)− + 2Au(NP) + 2L2(ac)− | (3) |
The presence of Au(I) in the reactive solutions with high concentrations of bromide ions was further confirmed by Raman analysis. Fig. 2 displays the Raman spectrum of an AuBr4− solution (red line) and the Raman spectrum from a reactive solution with high bromide ion concentration (black line). The AuBr4− is identified by two peaks located at 197 cm−1 and 212 cm−1.40–43 In the reactive solution, AuBr4− peaks are not present, as expected from the UV-Vis spectroscopic analysis, but a peak at 210 cm−1 is observed, which is associated with the presence of AuBr2−.43,44 The formation of Au(I) and the absence of Au(0) species for high bromide ion concentrations indicates that while reaction (1) takes place, the nucleation of AuNPs by reactions (2) and/or (3) is somehow inhibited under these conditions. The overall trend indicates that as the bromide ion concentration increases, the Au(0) nucleation is quenched, leading to the formation of larger AuNPs for intermediate concentrations and to the formation of Au(I) for high concentrations.
 | ||
| Fig. 2 Raman spectra of AuBr4− solution (red line) and of AuBr2− extracted from a reactive solution consisting of 0.1 mM HAuCl4, 50 mM KBr and 0.15 mM H2Q (black line). | ||
50 mM KBr reactive solution does not show spectral evidence of Au(0) nucleation over a period of 165 days; however, a year later, the reactive solution exhibits gold macroscopic crystals (data not shown). Furthermore, the Nernst potential calculated for the Au(I) to Au(0) reduction reaction (2), in the conditions of the reactive solution, predicts spontaneous Au(0) formation (ΔE = 0.52 V). It is clear that Au(0) formation in such conditions is thermodynamically favored; thus, the absence of AuNPs at relatively short times is due to the kinetic inhibition of the nucleation process. In this context, the solutions are called metastable.
A similar behavior to that observed with bromide ions is obtained for reactive solutions with variable concentrations of chloride ions and iodide ions (see Fig. S1 and S2, ESI†). Furthermore, the halide concentration necessary to obtain metastability, in similar conditions (HAuCl4 0.1 mM and H2Q 0.15 mM), follows the order [I−] < [Br−] < [Cl−] with values of 0.5 mM, 5 mM and 50 mM, respectively. Interestingly, the more stable the AuX2− complex (X = Cl, Br, I), the lower is the halide ion concentration needed to attain metastability. The formation of Au(I) metastable species in the presence of iodide ions has also been proposed in similar conditions to the ones studied here.45 It should be mentioned that when iodide ions are added to Au(III) solution, along with the ligand exchange reaction, triiodide ion is also formed due to the oxidation of iodide ion caused by the Au(III) species; this reaction was observed in our experiments and has already been reported.38,45 Nevertheless, with the addition of hydroquinone, the triiodide ion returns to the reduced iodide ion form, and thus the reactive solutions containing different halide ions are comparable.
Temperature effect
Because nucleation inhibition is kinetically controlled, it is interesting to explore how temperature affects the metastability in a reactive solution. To this end, a reactive solution containing 50 mM of KBr was prepared at 98 °C. Under these conditions, nucleation inhibition is not observed (see Fig. S3, ESI†) because gold microparticles are formed. Reactive solutions with identical compositions prepared at room temperature display metastability for months (see previous section). This indicates that the temperature accelerates the nucleation, diminishing the metastability period, and further confirms that nucleation inhibition by halide ions is a kinetically controlled phenomenon.Solution pH effect
Because hydroxyl ion is also a ligand for Au(III), the effect of halide ions should be evaluated considering the pH of the solution. Fig. 3 exhibits the spectral profiles of Au(III) complexes formed in 50 mM KBr solutions at different pH values (solid lines). The AuBr4− spectral profile at pH 6.5 (Fig. 3, solid black line) presents the characteristic features already described for Fig. 1a. As the pH increases, extinction profiles with decreasing intensity in the 300–500 nm interval are observed (red and green solid lines), while the peak at 382 nm is absent. This contrasting spectroscopic behavior corresponds to the presence of different relative concentrations of Au(III) complexes: AuBr4−, AuBr3(OH)−, AuBr2(OH)2−, AuBr(OH)3− and Au(OH)4−.38 At low pH values (pH = 6.5 or lower), AuBr4− predominates; however, as pH increases, bromide ions are partially replaced by hydroxyl ions in the coordination sphere of gold, which causes the peak at 382 nm to vanish. When hydroquinone is added to these solutions, notable differences are observed in the spectroscopic behavior (Fig. 3 dashed lines). At low pH values, where AuBr4− predominates (black line), the partial reduction of Au(III) to Au(I) is observed, and thus metastability is obtained, as was already discussed for Fig. 1. At pH values higher than 6.5, irrespective of the high bromide ion concentration, the solutions show a non-zero extinction value in the entire visible range, associated with the dispersion of large AuNPs.Although the Gibbs free energy is negative for Au(0) formation in the three cases evaluated here, the increase in hydroxyl concentration in the media clearly accelerates the nucleation process, diminishing the metastability. It is important to notice that the effect of increasing the pH is similar to the effect of lowering the halide ion concentration; in both the cases, the metastability period is reduced. It would appear that the presence of hydroxyl ions in the coordination sphere of Au(I) accelerates the nucleation process, although to prove this hypothesis, a deeper insight into the nucleation mechanisms beyond the classical theory is needed.
Reducing agent effect
To assess whether the quenching of AuNPs nucleation with increasing halide concentration is exclusive to H2Q, experiments under similar conditions were performed with ascorbic acid (AA). This reducing agent was chosen because it is the most employed agent in the seed-mediated growth method developed by Murphy's group,25–27 where the growth baths have similar conditions to those explored in this work (high halide concentrations), although hydroquinone has been recently used to produce gold nanorods by this method.46Fig. 4 shows the extinction spectra of reactive solutions prepared with AA and different halide ion contents. In the absence of halide ions (black line), the characteristic SPR peak of AuNPs around 520 nm is observed, indicating that AA is a strong enough reducing agent to nucleate the AuNPs. When the reactive solutions contain high concentrations of halide ions (green and red lines), the absence of spectral features associated with Au(III) and Au(0) species indicates the inhibition of AuNPs nucleation and the formation of Au(I) species. Therefore, nucleation quenching by halide ions is a more general behaviour, instead of a particular feature associated with a specific reducing agent. In this context, nucleation quenching should always be considered when AuNPs are formed by chemical reduction in the presence of halide ions.In the particular case of the quaternary ammonium-based seed-mediated method, the growth baths have high halide ion concentrations (typically around 0.1 M) originating from the quaternary ammonium salt employed (CTAB and CTAC). Therefore, it is most likely that the absence of self-nucleation during AuNPs growth using this method is a result of nucleation inhibition by halide ions. To prove this point, a simple test was performed, where the halide ions in the hexadecyltrimethylammonium salt were replaced by nitrate ions (see Fig. S4, ESI†). In this test, the reactive solution was identical in composition to a typical growth bath in the quaternary ammonium-based seed-mediated method, with the exception that halide ions were not present. In this reactive solution, no nucleation inhibition was observed whatsoever, indicating that halide ions are essential to attain self-nucleation inhibition with this method. From these experiments, a new role of the halide ions was identified in the quaternary ammonium-based seed-mediated growth method. As stated in the introduction, there are plenty of studies that show that halide ions play an important role in the anisotropic growth of AuNPs.36 We have now also determined that halide ions are responsible for preventing self-nucleation during AuNPs growth on this method.
Seed-mediated approach
The kinetic inhibition of nucleation in reactive solutions with high halide concentrations constitutes a basis for a seed-mediated approach. In these metastable solutions, homogeneous nucleation of AuNPs does not occur, but the Au(I) to Au(0) reduction (reactions (2) and/or (3)) may be activated by applying a perturbation. This perturbation may be the surface of previously synthesized AuNPs (seeds), which catalyze Au(0) formation, resulting in AuNPs growth, as represented in the scheme shown in Fig. 5a. When they are used to grow AuNPs, the metastable solutions are referred as growth baths. This section aims to illustrate this last point on how this basic recipe for metastability can be applied to grow AuNPs.The AuNPs employed as initial seeds have a spherical shape, with a mean diameter of 7 nm and a narrow size distribution (14% S.D.). The synthesis of these gold seeds is highly reproducible, and the AuNPs remain stable for months. The black line in Fig. 5b shows the spectrum of the solution of seeds with the same dilution they have after their addition to a growth bath; this can be taken as a reference spectrum at zero-time reaction after seeding. The gray line shows the final spectrum after the first step of seeding in a growth bath formed by iodide ions; no spectral changes were detected whatsoever after three minutes of seeding. There are two main features observed in the spectral evolution: (i) an increase in the extinction values and (ii) a red-shift in the SPR maximum from 514 nm to ca. 525 nm. Both characteristics are consistent with AuNPs growth, which is the only process that can occur given that the nucleation is inhibited. Similar spectroscopic characteristics were observed after every seeding process, indicating that the growth process took place at each step. The corresponding TEM images for the seeds and the AuNPs grown in the first and second steps of the seeding procedure with iodide ion growth baths are shown in Fig. 6a–c. The TEM images reveal that the AuNPs increase in size after each seeding step; this feature is more clearly shown in the size distributions represented in Fig. 6d. The AuNPs grown have a nearly spherical shape with sizes of (16 ± 3) nm and (37 ± 4) nm in diameter after the first and second steps of the seeding procedure, respectively. The absence of considerably smaller AuNPs confirms that the continuous nucleation process does not occur. The TEM results are in complete agreement with the main conclusions drawn from the spectroscopic analysis. It is notable that the size dispersion does not show a trend of increasing with the size of the grown AuNPs; values of 14%, 18% and 11% were obtained for the seeds and the 16 nm and 36 nm AuNPs, respectively. This indicates a control over the growth process, and it also provides evidence that the nucleation reaction is inhibited. As shown in Fig. 6, control over the AuNPs size can be achieved via the [Au(I)]/[seeds] ratio used in the seeding, as well as the seed size. These experiments demonstrate that reactions (2) and/or (3) are catalyzed on the surface of the AuNPs and constitute one of the many evidence that autocatalytic growth is present in the formation of metal NPs. Although no appreciable amount of Au(III) was detected during the growth of the AuNPs, Au(I) disproportionation by reaction (3) was considered because it has been reported to occur in conditions similar to those explored here.45 Furthermore, other reactions may be also taking place during AuNPs growth such as the oxidative etching of the AuNPs by Au(III) produced by the disproportionation reaction and the reduction of Au(I) by the iodide ions.
The AuNPs synthesized here offer the great advantage of being only stabilized by halide ions, and consequently they can be used in many applications without the need of further purification. Moreover, they can be easily functionalized with a broad variety of chemical compounds of interest. This synthetic procedure is also simple, inexpensive and environmentally friendly. The basic synthetic scheme of gold precursor (Au(III)), high halide ion concentration and a mild reducing agent can be modified as needed: shape inductors such as silver can be added to obtain anisotropic AuNPs as in the case of quaternary ammonium-based seed-mediated growth;27,28,30 the reducing agent can be varied, altering the Au(I) reduction rate, and organic molecules such as polymers or amphiphilic compounds can be added to increase the stability of the NPs, among other modifications. For example, although a different approach than that presented here was used, the suppression of AuNPs nucleation by iodide ions and its use for the growth of AuNPs with AA and poly(vinyl pyrrolidone) (PVP) has been reported.47
To further highlight the versatility of the seed-mediated approach presented in this study, Fig. 7 shows TEM results of the synthesized AuNPs using bromide ion growth baths in the presence of PVP, a biocompatible polymer which improves the stability of AuNPs. The AuNPs grown in this case have a spherical shape with sizes of (21 ± 2) nm and (49 ± 4) nm for the first and second steps of the seeding procedure, respectively. Similar to the AuNPs produced with iodide ion growth baths, the size dispersion in both cases does not exceed 10%, a feature indicative of remarkable growth control. Furthermore, some of the AuNPs that are grown in the presence of PVP exhibit considerably lower contrast than the spherical AuNPs of similar size, as shown in the TEM images. The lower contrast indicates that a small portion of the AuNPs have plate-like shapes. The origin of such anisotropic NPs is difficult to identify, given that anisotropic growth is still not well understood; however, it is worth to mention that such plate-like AuNPs have been already identified in aqueous environments in the presence of PVP.48 The obtained anisotropic NPs are predominantly triangular in the first step and hexagonal in the second step of the seeding procedure. In both the cases, they represent approximately 6% of the total. This strongly suggests that the AuNPs produced in the second step have grown from the anisotropic AuNPs formed in the first step of the seeding, and that no new anisotropic particles are formed in the final step. Therefore, it would appear that the triangles formed in the first step of the seeding evolved into the hexagons in the second step. A deeper insight into the effect of PVP and other species on the anisotropic growth of AuNPs constitutes a topic to be explored in the future.
Finally, Fig. 8 shows a comparison between the experimental diameter of the AuNPs with their corresponding dispersion vs. the predicted diameter after AuNPs growth for the four seeding procedures discussed above. The final diameter after the seeding was calculated as follows (see derivation in the ESI†):
 | ||
| Fig. 8 Experimental AuNPs diameter determined by TEM with corresponding dispersion bars vs. calculated diameter using the seed size and the [Au(I)]/[seeds] ratio as inputs. | ||
Experimental
Materials
All the stock solutions were prepared from AR chemicals and purified water (Milli RO, Milli Q system). HAuCl4 solutions were maintained in the dark to prevent their photochemical decomposition. NaBH4 solutions were prepared in ice-cooled water and were kept refrigerated for not more than one hour before their use.Reactive solutions
Aqueous solutions of 0.1 mM HAuCl4 in the presence of a reducing agent and variable concentrations of halide ions or hexadecyltrimethylammonium nitrate (CTAN) are referred to as reactive solutions. As reducers, 0.15 mM H2Q (hydroquinone, equinormal to Au(III)) and 0.15–1 mM ascorbic acid (AA, in excess compared to Au(III)) were employed. The concentrations of KCl, KBr and KI were varied between 0 and 50 mM. The pH was adjusted using NaOH. In all the cases, the reducing agent was added at the end. For the experiment performed at 98 °C, the solution was thermalized in a bath prior to the addition of the reducer. Then, the heating was suspended and the reactive solution was cooled with water to room temperature.Seed AuNPs
7 nm seed AuNPs were synthesized as follows: 1 mL of ice-cooled 0.1 M NaBH4 solution was added with vigorous stirring to 50 mL of 0.2 mM HAuCl4 and 2 mM HAc aqueous solution thermalized in a bath at 90 °C. This temperature was maintained for 30 min until the reaction was complete.Seed-mediated approach
The reactive solutions, which were used to grow the NPs, are called “growth baths”. Two different compositional growth baths were employed: (1) organic molecules-free iodide ion growth baths: 0.01 mM HAuCl4, 0.1 mM KI and 0.03 mM H2Q; (2) poly(vinyl pyrrolidone) (PVP) bromide ion growth baths: 0.05 mM HAuCl4, 20 mM KBr, 0.0145% PVP and 0.2 mM H2Q. For seeding, a two-step protocol was employed, where the volume ratio between the seeds and the growth bath solutions was kept at 0.1 and the growth bath composition was constant. In the first step, an aliquot of a previously prepared 1/10 dilution of the 7 nm seeds solution was added to a growth bath with vigorous stirring; the agitation was suspended after homogenization. Following the same procedure, in the second step, the AuNPs grown in the first step were used as seeds in a second seeding.Characterization
A Shimadzu UV-1200 spectrometer with a 1 cm quartz cell was used to characterize the solutions at room temperature. Transmission electron microscopy (TEM) characterization was carried out using a JEM-JEOL 1120 microscope. The samples were prepared, without any purification treatment, by seeding many drops of the colloidal solutions onto a Formvar-covered copper grid and evaporating the water in air at room temperature.Raman measurements
Raman spectra were recorded on a Horiba Jobin Yvon LabRAM HR Raman spectrometer using a He–Ne laser (632.82 nm) as the excitement source. Raman spectroscopy of AuBr4− was performed using a quartz cell containing an AuBr4− solution. To measure the AuBr2− spectrum, a reactive solution composed of 0.1 mM HAuCl4, 50 mM KBr and 0.15 mM H2Q was employed. Because the solution was too dilute to detect any Raman signals, it was necessary to concentrate the AuBr2−. To prevent the reaction of AuBr2− during the concentration step, the AuBr2− was extracted in CHCl3 employing CTAB as an extraction agent. Then, the CHCl3 was evaporated over a glass slide to produce thin layer deposits for Raman analysis.Conclusions
In the present work, it has been demonstrated that gold homogeneous nucleation by the chemical reduction of aqueous gold ions is kinetically quenched by the rise in the concentration of halide ions. The nucleation quenching increases as the AuX4− complex stability increases, where the halide ion concentration needed to attain nucleation inhibition follows the order: [Cl−] > [Br−] > [I−]. It is also observed that the nucleation quenching by halide ions decreases with increasing pH likely due to the changes in the speciation of the gold ions. Moreover, this effect of the halide ions on gold nucleation appears to be a general feature because it has been proven to be present with different reducing agents. Therefore, gold nucleation quenching should always be considered when halide ions are present. For example, this halide ion effect has been found to be responsible for the absence of self-nucleation in the quaternary ammonium-based seed-mediated growth method. Furthermore, halide ions can be used to manipulate and control the homogeneous nucleation of gold. We have shown that by selecting suitable conditions of pH and halide ion concentration, gold nucleation can be inhibited to generate Au(I) metastable species, which coexist together with the reducer. We have also proved that these metastable solutions constitute a basis for a seed-mediated approach. To achieve this, several metastable solutions were used as growth baths, where Au(I) reduction was activated on the surface of seed AuNPs, driving their growth. By adjusting the synthesis parameters, spherical shaped 7 nm AuNPs seeds have been grown to different sizes in a controlled and reproducible manner to as high as 49 nm. The AuNPs were synthesized under different conditions, without organic molecules and in the presence of PVP, a biocompatible polymer. Therefore, it has been shown that the seed-mediated approach proposed is highly reliable and that the synthesis conditions can be rationally designed to obtain the desired AuNPs sizes and surface traits among other properties.Acknowledgements
The authors acknowledge the financial support from the National Scientific and Technical Research Council of Argentina (CONICET), ANPCyT and SECyT. They also thank Dr Claudia Nome for her technical assistance in TEM microscopy.Notes and references
- P. Wang, B. Huang, Y. Dai and M.-H. Whangbo, Phys. Chem. Chem. Phys., 2012, 14, 9813 RSC.
- K. An and G. A. Somorjai, ChemCatChem, 2012, 4, 1512–1524 CrossRef CAS.
- R. R. Arvizo, S. Bhattacharyya, R. A. Kudgus, K. Giri, R. Bhattacharya and P. Mukherjee, Chem. Soc. Rev., 2012, 41, 2943 RSC.
- G. Doria, J. Conde, B. Veigas, L. Giestas, C. Almeida, M. Assunção, J. Rosa and P. V. Baptista, Sensors, 2012, 12, 1657–1687 CrossRef CAS PubMed.
- V. K. LaMer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 11 CrossRef.
- M. A. Watzky and R. G. Finke, J. Am. Chem. Soc., 1997, 119, 10382 CrossRef CAS.
- E. E. Finney and R. G. Finke, J. Colloid Interface Sci., 2008, 317, 351 CrossRef CAS PubMed.
- S. Papp, R. Patakfalvi and I. Dékány, Croat. Chem. Acta, 2007, 80, 493 CAS.
- R. Patakfalvi, S. Papp and I. Dékány, J. Nanopart. Res., 2007, 9, 353 CrossRef CAS.
- M. A. Pérez, R. Moiraghi, E. A. Coronado and V. A. Macagno, Cryst. Growth Des., 2008, 8, 1377 Search PubMed.
- V. V. Tatarchuk, A. P. Sergievskaya, I. A. Druzhinina and V. I. Zaikovsky, J. Nanopart. Res., 2011, 13, 4997 CrossRef CAS.
- B. Streszewskia, W. Jaworskia, K. Pacławskia, E. Csapó, I. Dékány and K. Fitzner, Colloids Surf., A, 2012, 397, 63 CrossRef PubMed.
- Y. Zhou, H. Wang, W. Lin, L. Lin, Y. Gao, F. Yang, M. Du, W. Fang, J. Huang, D. Sun and Q. Li, J. Colloid Interface Sci., 2013, 407, 8 CrossRef CAS PubMed.
- F. Wang, V. N. Richards, S. P. Shields and W. E. Buhro, Chem. Mater., 2014, 26, 5 CrossRef CAS.
- P. L. Redmond, A. J. Hallock and L. E. Brus, Nano Lett., 2005, 5, 131 CrossRef CAS PubMed.
- S. T. Gentry, S. F. Kendra and M. W. Bezpalko, J. Phys. Chem. C, 2011, 115, 12736 CAS.
- D. S. Sidhaye and B. L. Prasad, New J. Chem., 2011, 35, 755 RSC.
- Y. Zheng, J. Zeng, A. Ruditskiy, M. Liu and Y. Xia, Chem. Mater., 2014, 26, 22 CrossRef CAS.
- M. A. Pérez, in Recent Advances in Nanoscience, ed. M. M. Mariscal and S. A. Dassie, Research Signpost, Trivandrum, Kerala, India, 2007, ch. 6, p. 143 Search PubMed.
- M. Brust, J. Fink, D. Bethell, D. J. Schiffrin and C. J. Kiely, J. Chem. Soc., Chem. Commun., 1995, 1655 RSC.
- C. M. Donegá, P. Liljeroth and D. Vanmaekelbergh, Small, 2005, 12, 1152 CrossRef PubMed.
- C. Gao, J. Goebl and Y. Yin, J. Mater. Chem. C, 2013, 1, 3898 RSC.
- W. Niu, L. Zhang and G. Xu, Nanoscale, 2013, 5, 3172 RSC.
- C. Ziegler and A. Eychmüller, J. Phys. Chem. C, 2011, 115, 4502 CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- T. F. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648 CrossRef CAS PubMed.
- M. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2005, 109, 22192 CrossRef CAS PubMed.
- J. E. Millstone, S. Park, K. L. Shuford, L. Qin, G. C. Schatz and C. D. Mirkin, J. Am. Chem. Soc., 2005, 127, 5312 CrossRef CAS PubMed.
- M. Grzelczak, A. Sánchez-Iglesias, B. Rodríguez-González, R. Alvarez-Puebla, J. Pérez-Juste and L. M. Liz-Marzán, Adv. Funct. Mater., 2008, 18, 3780 CrossRef CAS.
- A. Sánchez, P. Díez, R. Villalonga, P. Martínez-Ruiz, M. Eguiláz, I. Fernández and J. M. Pingarrón, Dalton Trans., 2013, 42, 14309 RSC.
- B. D. Busbee, S. O. Obare and C. J. Murphy, Adv. Mater., 2003, 15, 414 CrossRef CAS.
- M. R. Langille, M. L. Personick, J. Zhang and C. A. Mirkin, J. Am. Chem. Soc., 2012, 134, 14542 CrossRef CAS PubMed.
- S. E. Lohse, N. D. Burrows, L. Scarabelli, L. M. Liz-Marzán and C. J. Murphy, Chem. Mater., 2014, 26, 34 CrossRef CAS.
- N. Almora-Barrios, G. Novell-Leruth, P. Whiting, L. M. Liz-Marzán and N. López, Nano Lett., 2014, 14, 871 CrossRef CAS PubMed.
- A. Usher, D. C. McPhail and J. Brugger, Geochim. Cosmochim. Acta, 2009, 73, 3359 CrossRef CAS PubMed.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef CAS.
- H. Stammreich and R. Forneris, Spectrochim. Acta, 1960, 16, 363 CrossRef CAS.
- P. L. Goggin and J. Mink, J. Chem. Soc., Dalton Trans., 1974, 1479 RSC.
- Y. M. Bosworth and R. J. H. Clark, J. Chem. Soc., Dalton Trans., 1975, 381 RSC.
- P. Pan and S. A. Wood, J. Solution Chem., 1993, 22, 163 CrossRef CAS.
- B. Braunstein, R. J. H. Clark, W. Ramsay, R. Foster and C. Ingold, J. Chem. Soc., Dalton Trans., 1973, 1845 RSC.
- A. K. Das and C. R. Raj, J. Phys. Chem. C, 2011, 115, 21041 CAS.
- L. Vigderman and E. Zubarev, Chem. Mater., 2013, 25, 1450 CrossRef CAS.
- C. Gao, J. Vuong, Q. Zhang, Y. Liu and Y. Yin, Nanoscale, 2012, 4, 2875 RSC.
- B. Lim, P. H. Camargo and Y. Xia, Langmuir, 2008, 24, 10437 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: UV-Vis characterization of reactive solutions formed with chloride and iodide ions and with CTAN. UV-Vis spectra of the effect of temperature on a reactive solution. Derivation of the equation used to predict the diameter of the AuNPs after seeding. See DOI: 10.1039/c4ra12003e |
| ‡ Current address: IFIR, Universidad Nacional de Rosario, Rosario, Argentina. |
| This journal is © The Royal Society of Chemistry 2015 |