Phase-dependent and defect-driven d0 ferromagnetism in undoped ZrO2 thin films
Abstract
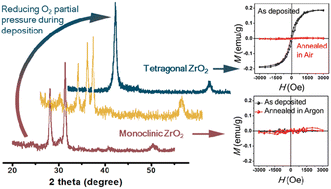
Undoped ZrO2 thin films are prepared on 〈100〉 Si substrates by reactive DC magnetron sputtering using a Zr target. By controlling the oxygen partial pressure during the deposition process, we can successfully control the phase structure of the as-deposited film, which can be tetragonal, monoclinic or a mixture of them. A magnetic property measurement reveals that phase-dependent d0 ferromagnetism exists in ZrO2 thin films. Specifically, only tetragonal ZrO2 thin films can be room-temperature ferromagnetic. Photoluminescence measurements, X-ray photoelectron spectroscopy analyses and post thermal annealing experiments suggest the d0 ferromagnetism in undoped tetragonal ZrO2 films is mainly driven by oxygen vacancies.

 Please wait while we load your content...
Please wait while we load your content...