A regular rippled pattern formed by the molecular self-organization of polyvinylpyrrolidone encapsulated Ag nanoparticles: a high transmissive coating for efficiency enhancement of c-Si solar cells†
Abstract
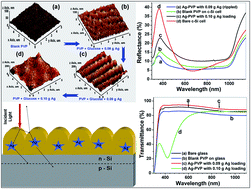
The formation, characterization and application of polyvinylpyrrolidone (PVP) encapsulated silver nanoparticles with a regular rippled pattern is reported. The metal precursor was reduced by an in situ reduction technique, where glucose was used as an organic and mild reducing agent to control the process. In order to create the rippled structured of the film by molecular self organization of the encapsulated Ag nanoparticles, the concentrations of the reactants were optimized. The structural properties of the deposited films were established by X-ray diffraction, transmission electron microscopy, atomic force microscopy and dynamic light scattering techniques. Atomic force microscopy (AFM) revealed a highly ordered ripple structure of the film, which originated due to the self organization of the Ag nanoparticles in the PVP matrix, whereas, transmission electron microscopy (TEM) revealed the core–shell structure of the Ag/PVP unit. UV-vis spectrophotometric analysis resulted in a broad absorption peak between 350 to 500 nm (centered at 418 nm), which is characteristic of Ag nanoparticles. A notable reduction in reflectance and an increase in transmittance, leading to an enhancement in the overall efficiency, were observed when the rippled pattern was spin-coated on a c-Si solar cell.

 Please wait while we load your content...
Please wait while we load your content...