Novel triethylamine catalyzed S → O acetyl migration reaction to generate candidate thiols for construction of topological and functional sulfur-containing polymers†
Gang Wang‡
ac,
Li Peng‡b,
Yaochen Zhengb,
Yanqin Gaob,
Xuedong Wua,
Tianhui Renc,
Chao Gao*b and
Jin Han*a
aKey Laboratory of Marine New Materials and Related Technology, Zhejiang Key Laboratory of Marine Materials and Protection Technology, Ningbo Institute of Material Technology & Engineering, Chinese Academy of Sciences, Ningbo, 315201, P. R. China. E-mail: hj@nimte.ac.cn
bMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027, P. R. China. E-mail: chaogao@zju.edu.cn
cSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, P. R. China
First published on 15th December 2014
Abstract
We describe a novel triethylamine catalyzed S → O acetyl migration reaction for yielding thiol compounds under mild conditions through the formation of a transitional 5-membered ring. A series of epoxy compounds have been transformed into their thiol counterparts which could be used for construction of topological and functional sulfur-containing polymers. The one-pot two-step processes including the S → O acetyl migration and the following thiol-click reactions avoided separation of thiol intermediates. Applying these processes on a new-type latent polythiols overcomes crosslinking problem usually met in preparation of multithiol compounds due to the formation of disulfide bonds.
Introduction
Thiol compounds are very useful for polymer materials. Their related chemistry, especially thiol-click chemistry, including thiol-Michael addition, thiol–ene, thiol–yne, thiol–isocyanate, thiol–isothiocyanate and thiol–halogen reactions, has attracted immense scientific interest. Topological macromolecules with structures such as starlike, end-functional, comb, hyperbranched, dendrimer, and network have been constructed. Functional polymer materials like oxidation-responsive bio(nano)materials,1 polysulfide elastomers,2 fast curing agent for epoxy resins,3 degradable materials,4 high refractive index film,5 and liquid crystal polymers6 have been developed.Examples for construction of topological and functional polymers from thiol compounds via thiol-related chemistry are presented as follows. Hoyle and Lowe reported a convergent synthesis of 3-arm star polymers from monothiol terminated poly(N,N-diethylacrylamide) via a thiol-Michael click reaction.7 Ji et al. prepared thiol terminated poly(ethylene glycol) to modify vinyl group functionalized Si wafers.8 Haddleton et al. opened S–S bonds at the surface of salmon calcitonin to generate thiol groups for further graft with poly(ethylene glycol)arylates.9 Klemm et al. used dithiols to prepare highly refractive polythiourethane for fabricating optic materials.10 Oğuz Türünc et al. and Han et al. used a thiol–ene click reaction to prepare comb-structured polysulfides with various side groups.11 Benzhong Tang et al. used aromatic diynes and dithiols to prepare electronically active conjugated poly(vinylene sulfide)s.12 Several teams have prepared sulphur containing hyperbranched polymers and dendrimers.13 Perrier and coworkers first reported the synthesis of functional hyperbranched polymers by photo-initiated thiol–yne click polymerization of AB2 telechelic polymer.13a Casado et al. reported the synthesis of a carbosilane dendrimer with 36 terminal thiol groups, and investigated its interaction with transitional metal ions.13b Hawker et al. used two orthogonal and efficient reactions—‘epoxy–amine’ and ‘thiol–ene’ coupling for rapid growth of the dendritic scaffold.13c Qin Li et al. prepared crosslinked polythiourethane elastomers which had much higher refractive index values than polyurethane counterparts.14 Bowman et al. utilized multithiol compounds and vinyl-containing liquid preceramic monomers to manufacture highly cross-linked polymer networks with various shapes.15 Vo et al. used various thiol monomers to prepare oxidation-responsive polysulfides for anti-inflammatory therapies.16
These applications strongly depend on the thiol compound used and urgently call for synthetic methods of thiol compounds developing towards rapidness, mildness, high-yield and scale-up. By now, many strategies have been developed using various sulfur resources such as SC(NH2)2, CH3COSH, H2S, NCS–, NaHS, Na2S2O3, CS2 and P2S5.17 However, multistep, relatively low yields, harsh conditions, and sulfide/disulfide byproducts still trouble industry and laboratory synthesis.
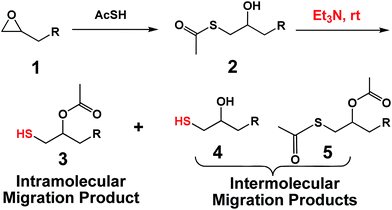
S → O acetyl migration reaction (SOAM) for preparing thiols was first discovered by Sjöberg using sodium carbonate as early as 1941, but little attentions has been paid to develop it since then (Table 1).18a In 1952, Miles et al. reported that acetic acid could catalyze SOAM at 100 °C affording thiols in a low yield of 18.5% in 13 h, but our recent experiment proved that the reaction still achieved the yield just by heating at 100 °C.18b In 1988, Ward et al. used pyridine to achieve a yield up to 86% in 10 weeks.18c These unsatisfactory results made SOAM fail to become a common used method. It was speculated that weak alkaline resulted in slow rate while strong alkaline led to removal of acetyl groups and generation of sulfide or disulfide byproducts, so an appropriate alkali should be searched out. Herein, we reported that triethylamine (TEA) was able to fulfill the reaction demands including rapidness, mildness, high yield (>86%) and scale-up. TEA catalyzed SOAM was composed of major intramolecular reaction and minor intermolecular transthioesterification, which was reported for the first time. Because several thiol-click reactions also adopted TEA as catalyst, the combination with SOAM gave a series of very useful one-pot two-step processes.
Experimental section
Chemicals
Allyl glycidyl ether, 1,6-diisocyanatohexane, n-butyl acrylate, propylene oxide, methyl acrylate, propargyl alcohol, thiolacetic acid, N-isopropylacrylamide, glycidyl methacrylate, 2,2′-azobisisobutyronitrile, 4-dimethylamino-pyridine (DMAP), 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) and 2,2-dimethoxy-2-phenylacetophenone were purchased from Aladdin Chemical Co., China. Propargyl glycidyl ether was obtained from DaTang Pharmacy Co., China. Allyl isocyanate and ally isothiocyanate were acquired from Sigma Aldrich. 1-Octyne and triglycidyl isocyanurate were purchased from TCI Shanghai. Octavinyl-T8-silsesquioxane was purchased from Hybrid Plastics, Co. Epoxy terminated polydimethylsiloxane (PDMS) (Mn = 2300) was supplied by Evonik Co. Triethylamine (TEA), CH2Cl2, CHCl3, tetrahydrofuran, methanol, toluene, and diethyl ether were purchased from Sinopharm Chemical Reagent Co., Ltd. Tetrahydrofuran and toluene were dried over CaH2 and distilled. All other reagents were used without further purification.Measurements
1H and 13C nuclear magnetic resonance spectroscopy (NMR) spectroscopy was carried out on a Varian Mercury plus 400 NMR spectrometer at 20 °C. The samples were dissolved with CDCl3 and the solutions were measured with tetramethylsilane as an internal reference. Fourier transform infrared (FTIR) spectra were recorded on a PE Paragon 1000 spectrometer (film or KBr disk). Gel permeation chromatography (GPC) was recorded on a Perkin Elmer HP 1100, using THF as the eluent at a flow rate of 1 mL min−1, RI-WAT 150 CVt+ as the detector and linear polystyrene for calibration at 40 °C for characterization of apparent molecular weights. Mass spectrum was record by Bruker Esquire 3000 plus ion trap mass spectrometer (Bruker-Franzen Analytik GmbH, Bremen, Germany). Nitrogen was used as nebulizing gas at a pressure of 10 psi and drying gas at a flow rate of 5 L min−1. The drying gas temperature was set at 250 °C and the capillary voltage was set at 4000 V. Solutions were infused to the mass spectrometer with a syringe pump at a flow rate of 6 μL min−1.General procedure for thiol–epoxy reactions between epoxy compounds 1 and thioacetic acid to generate 2
A CH2Cl2 (12 mL) solution of thioacetic acid (1.84 g, 24.0 mmol) was mixed with a CH2Cl2 solution of epoxy compounds (20.0 mmol). Then, TEA (100.0 mg, 1.0 mmol) was injected into the mixture through a microsyringe. After vigorous stirring for 10 h at room temperature, the mixture was diluted with another 40 mL of CH2Cl2, washed with 1 M HCl aqueous solution, saturated NaHCO3 aqueous solution, and deionized water, successively, dried by anhydrous MgSO4. All the volatiles were evaporated to afford 2 in nearly quantitative yield.General procedure for SOAM reaction to transform 2 into 3
The prepared 2 (5.0 mmol) was dissolved in 16 mL of CHCl3. Then, the solution was added dropwise into a mixture of CHCl3 (30 mL) and TEA (6 mL) under N2 atmosphere at a concentration of 0.1 mol L−1. After vigorous stirring for 6 h at 25 °C, the reaction mixture was evaporated on a rotary evaporation. The residual was diluted with diethyl ether, washed with 1 M HCl aqueous solution to remove residual TEA and deionized water, successively, dried by anhydrous MgSO4, and finally evaporated to afford 3 as a major product and 4 and 5 as minor products. The molar ratios of 3, 4, and 5 in the products could be obtained by comparing the integrations of proton signals of –SCOCH3 and –OCOCH3. Pure products, 3a–3d, could be acquired be flash chromatography. The products derived from 1e–1g were mixtures. The yields were presented in Table 2.| Epoxy compounds | Yield (%) | |||
|---|---|---|---|---|
| 3 | 4 | Total thiol groups (3 + 4) | 5 | |
| a For 1e–1g containing multi epoxy groups, the products were mixtures and the yields of 3, 4 and 5 were calculated according to the integrations of proton signals of –SCOCH3 (2.34 ppm) and –OCOCH3 (2.06 ppm) in the 1H NMR spectra. | ||||
| 1a | 90.8 | 4.6 | 95.4 | 4.6 |
| 1b | 88.2 | 5.9 | 94.1 | 5.9 |
| 1c | 89.8 | 5.1 | 94.9 | 5.1 |
| 1d | 89.6 | 5.2 | 94.8 | 5.2 |
| 1ea | 94.2 | 2.9 | 97.1 | 2.9 |
| 1fa | 86.8 | 6.6 | 93.4 | 6.6 |
| 1ga | 85.8 | 7.1 | 92.9 | 7.1 |
Diluted solution and slow addition operation for improving the yield of 3a from 2a
2a (1.0 g, 5.2 mmol) was dissolved in 4 mL of CHCl3 and the solution was added very slowly into a mixture of CHCl3 (150 mL) and TEA (30 mL) in a speed of 1 mL h−1 by using a Laboratorial Syringe Pump (Baoding Longer Precision Pump Co., Ltd). After the addition, the reaction system was stirred for another 2 h. All the volatiles were removed under reduced pressure. The residual was diluted with diethyl ether, washed with 1 M HCl aqueous solution to remove residual TEA and deionized water, successively, dried by anhydrous MgSO4, and finally evaporated. Before applying flash chromatography, the residual was characterized by 1H NMR. By comparing the integrations of proton signals of –SCOCH3 and –OCOCH3, the yield of 3a was above 97% (more experimental details on one-pot two-step processes are presented in ESI†).Results and discussion
As shown in Scheme 1, SOAM reaction converted a series of epoxy compounds into thiol compounds. Thus, a toolbox of various candidate thiols is presented to the polymer science community for future use. Monomer 3a was an AB monomer and could be used to prepare linear polysulfide via thiol–ene click polymerization. Monomer 3c was an AB2 monomer, and could be used to synthesize hyperbranched polysulfide. 3d combines a methacrylate group for polymerization and a thiol group for further modification. Three-arm thiol monomer 3f can probably serve as fast curing agent for epoxy resins, crosslinking agent for polysulfide elastomers. Preparation of 1e provided a novel route for generating thiol telechelic polymers. Polythiol 3g containing numerous thiol groups can probably be used to afford comb polymer via thiol-Michael, thiol–isocyanate and thiol–epoxy click reaction.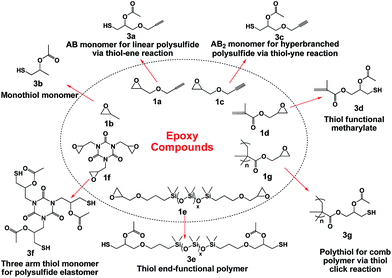 | ||
| Scheme 1 Various sulphur-containing monomers and polymer materials transformed from different epoxy compounds. | ||
Scheme 2 presented the detailed procedures of transformation of epoxy compounds into thiol compounds. The β-hydroxy thioacetates 2 were quantitatively derived from the thiol–epoxy reactions between epoxy compounds and thioacetic acid. The reaction could take place in water or by using catalytic amount of TEA (1.0 mol%).19 The subsequent intramolecular SOAM in a TEA–solvent mixture (volume ratio: 1/5–1/1) at a concentration of 0.1 mol L−1 could rapidly transform 2 into β-acetate thiols 3 via an intramolecular mode. β-Hydroxy thiols 4 and diacetates 5 were also isolated, indicating that a simultaneous intermolecular migration reaction also took place. The total yield of thiol compounds comprised of 3 and 4 was above 92.9%. The S → O acetyl migration reaction from 2 to 3 can take place in CH2Cl2, CHCl3, THF and methanol. DMAP and DBU were also tested for the intramolecular SOAM reaction (Table S1†). Although much less amounts of catalysts were employed, the amounts of by-product 5 increased. In general, although intermolecular migration gives some β-hydroxylthiol groups, the SOAM still provides useful thiol compounds or resins due to the high total thiol yield. We believe that the most suitable application area of SOAM is polymer science.
Based on the previous investigations, the proposed mechanisms of SOAM were presented in Scheme 3.20 The intramolecular migration of acetyl group starts from the abstraction of the proton from the hydroxyl group of I by TEA to give an unstable alkoxide ion II. Then, the anion attacks the carbonyl carbon atom to form a transitional 5-membered ring III. Because sulfur is easier to be polarized than oxygen, the ring opening occurs more often with the cleavage of S–C bonds than that of O–C bonds. The resulted IV is more stable than II, which could also be demonstrated by the fact that the reaction between 2-hydroxy-1-ethanethiol and propargyl bromide under alkaline condition gave a thioether rather than ether.21 The coproducts of VII and IX should result from an intermolecular acetyl migration process. The reaction of II with I does not seems to be responsible, because the highly active alkoxide ion tends to attack the intramolecular carbonyl carbon atom rather than waiting for a collision chance to carry out the intermolecular migration. Besides, the experimental result of SOAM reaction in methanolic solution also supported this judgment that was, the yield of V was nearly the same as that in dichloromethane solution and the yield of IX did not increase. A mechanism of transthioesterification reaction between IV and I makes sense. It was reported that transthioesterification could proceed relatively quickly and reversibly in the presence of alkaline and involved in fatty acid biosynthesis, native chemical ligation peptide coupling methodology, and polythioester synthesis.22 IV possesses strongly nucleophilic sulfide ions. The transthioesterification begins with the attack of IV towards I, crosses a transitional state VI, and finally affords VII and IX.
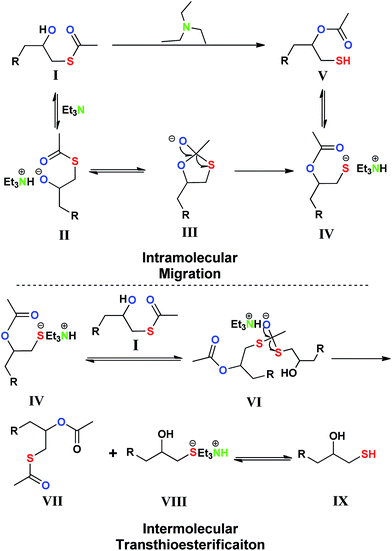 | ||
| Scheme 3 Proposed mechanisms of intramolecular migration and intermolecular transthioesterification. | ||
Transthioesterification reaction needs the collision between IV and I molecules, so decreasing the concentration of the reaction mixture to reduce their collision frequency should inhibit the intermolecular migration. Highly diluted solution and slow addition have been used to suppress intermolecular reaction in preparation of cyclic polymers from α,ω-functional telechelic polymers.23 In our experiment, a CHCl3 (4 mL) solution of 2a (1.0 g, 5.2 mmol) was added to a mixture of CHCl3 (150 mL) and TEA (30 mL) in a speed of 1 mL h−1 to give 3a in the yield of above 97%.
To test the reaction activity of the thiol compounds generated by SOAM, five typical compounds were chosen, such as methyl acrylate (MA), hexamethylene diisocyanate (HDI), propargyl alcohol, 1-octyne, and octavinyl-T8-silsesquioxane (Scheme S1, ESI†). A series of thiol-click chemistry including ionic and radical types were carried out under mild conditions, such as thiol-Michael addition, thiol–isocyanate, thiol–ene, and thiol–yne. The products were confirmed by NMR and MS (ESI†). All the yields were quantitative, so the thiyl radicals and thiolate ions of these thiols possesses excellent activity. The β-acetate groups don't seem to have a huge influence on the activity of thiol groups. Besides, the addition product from propargyl alcohol is interesting, because it is not easy to obtain a compound owning a primary alcohol and acetyl-protected secondary alcohols.
Because TEA can also catalyze ionic types of thiol-click reactions,20a we attempted to conduct SOAM and thiol-click reactions within one pot. For instance, as shown in Scheme 4, a methanolic solution of 2a and n-butyl acrylate was added dropwise to a TEA–methanol (1/1) mixture, and the final product was obtained after reacting several hours. These one-pot two-step processes were successfully carried out employing thiol-Michael addition, thiol–isocyanate, and thiol–isothiocyanate. Separation of intermediate thiol compounds was avoided and the final yields were generally above 81%. It was believed that these one-pot two-step processes will greatly promote applications of thiol-click reactions.
 | ||
| Scheme 4 One-pot two-step processes employing three types of thiol-click reactions and 2a as a model compound. | ||
Macromolecules containing high density of thiol groups have been reported,24 whereas further applications were rarely done because they were liable to crosslink into insoluble networks through the unfavorable formation of disulfide bonds.13b In this work, although polythiols 1g was successfully attained, it suffered from crosslinking in less than 1 day. In order to solve this problem, a latent polythiols was announced (Scheme 5). It was prepared via free radical polymerization of 2d. The thiol groups were totally protected by acetyl groups before use. When in use, the one-pot two-step processes could be employed as shown in Scheme 5, during which the thiols groups were generated in situ. In this way, methyl acrylate, N-isopropylacrylamide, allyl isocyanate, and allyl isothiocyanate were successfully grafted. It was believed that this novel latent polythiols technology furnished a novel method for polymer postfunctionalization and paved the way for the application of polythiols in thermosetting plastics and coatings. Moreover, we have successfully used the latent polythiols as anti-corrosion primer to form a thick and compact absorption film on copper, and the results will be published elsewhere.25
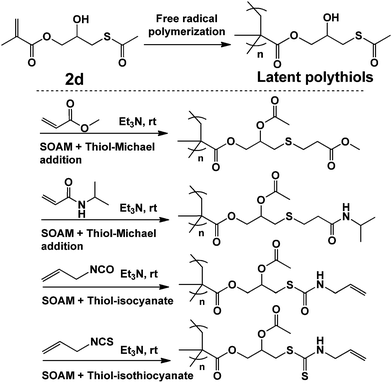 | ||
| Scheme 5 Synthesis of the novel latent polythiols and the postfunctionalization employing one-pot two-step processes. | ||
Conclusions
In conclusion, we presented a general strategy for facile synthesis of thiol compounds by TEA catalyzed intramolecular SOAM with total yield of thiol groups above 92%. The intramolecular migration goes through a transition stage with the formation of a 5-membered ring; the intermolecular migration probably adopts a transthioesterification mode. Various epoxy compounds and resins could be converted into thiols, inspiring new ideas and providing new materials for organic synthesis, thermosetting plastics and coatings. The one-pot two-step processes and the latent polythiols strategy exhibit great convenience and convincingly promote the engineering of thiol-click reactions. Despite these developments, the transthioesterification reaction must be inhibited to make SOAM more regioselective. A more suitable alkaline catalyst or an inhibition strategy by adding other cheap thiols (e.g. 1-propanethiol and 1,2-ethanedithiol) may help.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (20974093, 51173162, and 51303192), Ningbo Natural Science Foundation (2013A610018), Qianjiang Talent Foundation of Zhejiang Province (2010R10021), Fundamental Research Funds for the Central Universities (2011QNA4029), Research Fund for the Doctoral Program of Higher Education of China (20100101110049), and Zhejiang Provincial Natural Science Foundation of China (R4110175).Notes and references
- A. Napoli, M. Valentini, N. Tirelli, M. Müller and J. A. Hubbell, Nat. Mater., 2004, 3, 183 CrossRef CAS PubMed.
- E. Zaitseva and A. Donskoi, Polym. Sci., Ser. D, 2008, 1, 289 CrossRef.
- S. K. Reddy, N. B. Cramer, T. Cross, R. Raj and C. N. Bowman, Chem. Mater., 2003, 15, 4257 CrossRef CAS.
- A. E. Rydholm, C. N. Bowman and K. S. Anseth, Biomaterials, 2005, 26, 4495 CrossRef CAS PubMed.
- C. Lu, Z. Cui, Z. Li, B. Yang and J. Shen, J. Mater. Chem., 2003, 13, 526 RSC.
- P.-P. Gilles, J.-C. Milano and J.-L. Vernet, Macromol. Chem. Phys., 2003, 204, 2222 CrossRef CAS.
- J. W. Chan, B. Yu, C. E. Hoyle and A. B. Lowe, Chem. Commun., 2008, 4959 RSC.
- M. Yang, J. Mao, W. Nie, Z. Dong, D. Wang, Z. Zhao and X. Ji, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2075 CrossRef CAS.
- M. W. Jones, G. Mantovani, S. M. Ryan, X. Wang, D. J. Brayden and D. M. Haddleton, Chem. Commun., 2009, 5272 RSC.
- E. Klemm and C. Stöckl, Makromol. Chem., Rapid Commun., 1991, 192, 153 CrossRef CAS.
- (a) O. Türünc and M. A. R. Meier, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1689 CrossRef; (b) J. Han, Y. Gao and C. Gao, Polym. Chem., 2012, 3, 1918 RSC.
- J. Liu, J. W. Y. Lam, C. K. W. Jim, J. C. Y. Ng, J. Shi, H. Su, K. F. Yeung, Y. Hong, M. Faisal, Y. Yu, K. S. Wong and B. Z. Tang, Macromolecules, 2010, 44, 68 CrossRef.
- (a) D. Konkolewicz, A. Gray-Weale and S. Perrier, J. Am. Chem. Soc., 2009, 131, 18075 CrossRef CAS PubMed; (b) J. A. Camerano, M. A. Casado, M. A. Ciriano, C. Tejel and L. A. Oro, Dalton Trans., 2005, 3092 RSC; (c) T. Kang, R. J. Amir, A. Khan, K. Ohshimizu, J. N. Hunt, K. Sivanandan, M. I. Montanez, M. Malkoch, M. Ueda and C. J. Hawker, Chem. Commun., 2010, 46, 1556 RSC.
- Q. Li, H. Zhou, D. A. Wicks, C. E. Hoyle, D. H. Magers and H. R. McAlexander, Macromolecules, 2009, 42, 1824 CrossRef CAS.
- S. K. Reddy, N. B. Cramer, T. Cross, R. Raj and C. N. Bowman, Chem. Mater., 2003, 15, 4257 CrossRef CAS.
- C. D. Vo, G. Kilcher and N. Tirelli, Macromol. Rapid Commun., 2009, 30, 299 CrossRef CAS PubMed.
- R. J. W. Cremlyn, An introduction to organosulfur chemistry, Wiley, New York, 1996 Search PubMed.
- (a) B. Sjöberg, Ber. Dtsch. Chem. Ges. A, 1942, 75, 13 CrossRef; (b) L. W. C. Miles and L. N. Owen, J. Chem. Soc., 1952, 817 RSC; (c) J. P. Ward, Chem. Phys. Lipids, 1988, 47, 217 CrossRef CAS.
- A. Z. Halimehjani, A. Jalali, M. Khalesi, A. Ashouri and K. Marjani, Synth. Commun., 2011, 41, 1638 CrossRef CAS.
- (a) L. Pavlova and F. Y. Rachinskii, Russ. Chem. Rev., 1968, 37, 587 CrossRef PubMed; (b) W. P. Jencks, S. Cordes and J. Carriuolo, J. Biol. Chem., 1960, 235, 3608 CAS; (c) R. B. Martin and R. I. Hedrick, J. Am. Chem. Soc., 1962, 84, 106 CrossRef CAS.
- J. Han, Y. Zheng, B. Zhao, S. Li, Y. Zhang and C. Gao, Sci. Rep., 2014, 4, 4387 Search PubMed.
- (a) S. Tallon, A. C. Lawlor and S. J. Connon, Arkivoc, 2011, 4, 115 CrossRef; (b) N. Weber, K. Bergander, E. Fehling, E. Klein, K. Vosmann and K. D. Mukherjee, Appl. Microbiol. Biotechnol., 2006, 70, 290 CrossRef CAS PubMed; (c) P. E. Dawson, T. W. Muir, I. Clark-Lewis and S. Kent, Science, 1994, 266, 776 CAS.
- (a) S. B. L. Vollrath, S. Brase and K. Kirshenbaum, Chem. Sci., 2012, 3, 2726 RSC; (b) B. A. Laurent and S. M. Grayson, Chem. Soc. Rev., 2009, 38, 2202 RSC.
- (a) M. Le Neindre and R. Nicolaÿ, Polym. Chem., 2014, 5, 4601 RSC; (b) E. Hrsic, H. Keul and M. Möller, Eur. Polym. J., 2012, 48, 761 CrossRef CAS PubMed; (c) M. Le Neindre and R. Nicolaÿ, Polym. Int., 2014, 63, 887 CrossRef CAS; (d) M. T. Gokmen and F. E. Du Prez, Prog. Polym. Sci., 2012, 37, 365 CrossRef CAS PubMed.
- L. Gu, J. Han and X. Wu, results to be published.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra, IR spectra, mass spectra, and GPC curves. See DOI: 10.1039/c4ra09842k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |