Hydrolytic polycondensation of diethoxydimethylsilane in carbonic acid†
A. A. Kalininaa,
I. V. Elmanovichb,
M. N. Temnikovab,
M. A. Pigalevabc,
A. S. Zhiltsovc,
M. O. Gallyamovbc and
A. M. Muzafarov*ab
aN. S. Enikolopov Institute of Synthetic Polymer Materials, Russian Academy of Sciences, 117393 Profsoyuznaya St. 70, Moscow, Russian Federation. E-mail: aziz@ineos.ac.ru
bA. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991 Vavilova St. 28, Moscow, Russian Federation
cFaculty of Physics, Lomonosov Moscow State University, 119991 Leninskie gory 1-2, Moscow, Russian Federation
First published on 10th December 2014
Abstract
A new process for producing silicones based on chlorine-free reagents is suggested. Carbonic acid at elevated pressure and temperature is shown to be an effective reagent for converting alkoxysilane to silicones. The process conditions make it possible to control the ratio between linear and cyclic products.
Silicones play a significant role in various areas of human activity, starting from cosmetic and homecare compositions and ending with materials for spaceship and aircraft construction, automotive and building industries. Most of these materials are produced by the so-called “chlorine cycle” consisting of chlorination and dechlorination of silicon by different means. Being widely used in a great amount, chlorine-containing reagents are one of the most important drawbacks that accompanies the manufacture of silicones in general.
Apparently, shifting to chlorine-free synthetic methods has become relevant direction and does not require any additional reasoning. Recently appeared papers devoted to the synthesis of polysiloxanes via the Piers–Rubinsztajn reaction,1–7 the condensation of alkoxysilanes in an active medium8,9 allow alkoxysilanes to be considered as alternative reagents for a large-scale production of polysiloxanes. Research into the interaction between alkoxysilanes and carbonic acid seems to be very promising in this context. Once processes carried out in supercritical CO2 have become widely established, the prospects of carbonic acid usage could be reassessed easily, using the same set of equipment, in particular, for producing polysiloxanes. Carbonic acid is unique because the equilibrium position (Scheme 1) can be effectively controlled by adjusting pressure. In turn, it allows the medium acidity to be controlled in a wide range, more specifically, from pH = 3.9 at 9 bar to pH = 2.8 at 200 bar.10–15
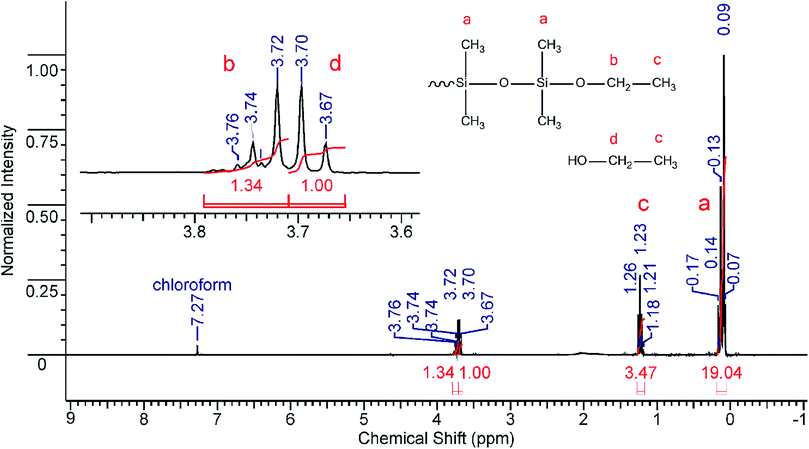
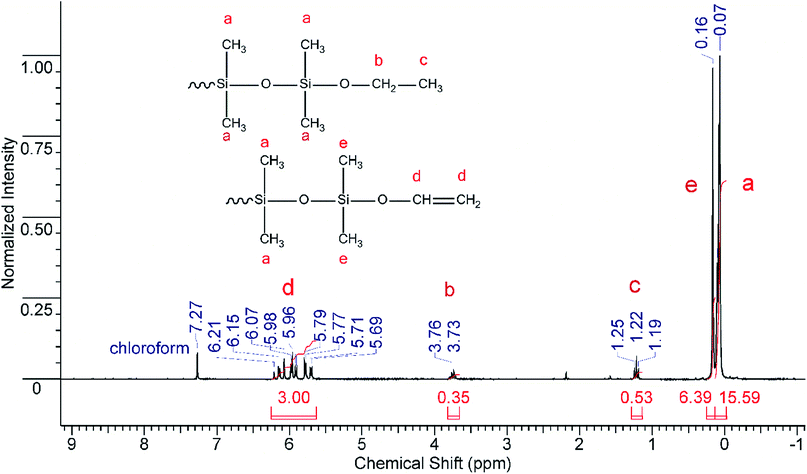
These features were used for the investigation of interaction between diethoxydimethylsilane and carbonic acid. The process was carried out in a high pressure sealed autoclave under various conditions (temperature and pressure). Diethoxydimethylsilane (10 mL) and deionized water (5 mL) were fed into the autoclave. Afterwards, liquid compressed CO2 was introduced at the selected pressure (150–350 bar). The desired temperature within the autoclave (20–110 °C) was set with an electronic thermostat. The reaction duration was varied from 10 minutes to 3 hours. When the excess pressure was released, the reaction mixture was analyzed by means of GLC, IR- and NMR-spectroscopy. The products obtained were fractionated and blocked with chlorodimethylvinylsilane that made it possible to determine both the content of hydroxyl groups and the ratio of cyclic to linear products.9 A gas–liquid chromatogram (GLC) of one reaction mixture (Table 1, entry 3) is presented in Fig. 1.
| Polycondensation conditions | Product properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | P1a (bar) | P2b (bar) | t (°C) | Duration (min) | Residue of OAlk (wt%) | OH-groups mass fraction (wt%) | Product composition (wt%) | Cycle/linear product ratio (wt%/wt%) | ||||
| D3 | D4 | D5 | D6 | Linear | ||||||||
| a Pressure at initial conditions.b Actual pressure after heating. | ||||||||||||
| 1 | 150 | 425 | 60 | 180 | 1.2 | 9.2 | 3 | 33 | 6 | 1 | 57 | 43/57 |
| 2 | 150 | 425 | 60 | 60 | 8.6 | 9.1 | 5 | 24 | 4 | 2 | 65 | 35/65 |
| 3 | 150 | 425 | 60 | 10 | 2.5 | 6.0 | 3 | 12 | 1 | 0 | 84 | 16/84 |
| 4 | 350 | 725 | 60 | 180 | 2.6 | 11.8 | 0 | 38 | 6 | 0 | 56 | 44/56 |
| 5 | 150 | 768 | 120 | 60 | 3.7 | 8.2 | 3 | 9 | 0 | 0 | 88 | 12/88 |
| 6 | 350 | 725 | 60 | 60 | 6.5 | 9.6 | 7 | 24 | 5 | 1 | 63 | 37/63 |
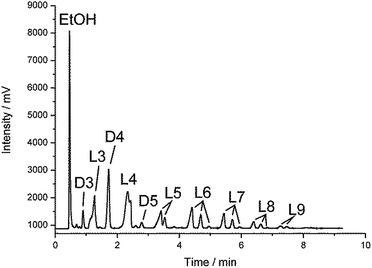 | ||
| Fig. 1 GLC-curve of the non-blocked reaction mixture.16 | ||
The reaction mixture components were identified using standards. The results were in good agreement with NMR data. Typical NMR spectra for the reaction mixture as well as for the blocked products are presented in Fig. 2 and 3, respectively. The analysis results are summarized in Table 1. Note that the interaction between diethoxydimethylsilane and carbonic acid is accompanied by the full monomer conversion as well as by the formation of ethanol and a mixture of dimethylcyclosiloxanes and linear oligomers having either hydroxyl or ethoxysilyl end groups. It means that the reaction of diethoxydimethylsilane with carbonic acid proceeds in accordance with the hydrolytic polycondensation mechanism. The yield of siloxane products is quantitative. During the investigation, it was found out that the conditions of polycondensation of diethoxydimethylsilane in carbonic acid medium significantly influence the composition of products. In all probability, this fact can be used to obtain polydimethylsiloxanes of pre-set desired structure. As an example: decreasing the process duration (Table 1; entries 1–3) as well as increasing temperature (Table 1; entries 5 and 6) favors formation of linear products; whereas, changes in pressure (Table 1; entries 1 and 4) provided that other parameters remain the same, do not result in significant alterations in the composition of the polycondensation products. Technological aspects of the reaction are quite optimistic. The process temperature and duration could be further optimized and could be considered as a promising basement for the continual process development. Obviously, further research into mechanism of every single stage and, especially, structure of intermediates is necessary. Nevertheless, the data obtained make it possible to conclude that a very promising brand-new method for producing polysiloxane products has been developed.
Acknowledgements
This work was financially supported by Russian Science Foundation (project no. 14-23-00231).Notes and references
- D. B. Thompson and M. A. Brook, J. Am. Chem. Soc., 2008, 130, 32 CrossRef CAS PubMed.
- J. Chojnowski, S. Rubinsztajn, J. Cella, W. Fortuniak, M. Cypryk, J. Kurjata and K. Kazmierski, Organometallics, 2005, 24, 6077 CrossRef CAS.
- J. Chojnowski, W. Fortuniak, J. Kurjata, S. Rubinsztajn and J. Cella, Macromolecules, 2006, 39, 3802 CrossRef CAS.
- J. Cella and S. Rubinsztajn, Macromolecules, 2008, 41, 6965 CrossRef CAS.
- J. Chojnowski, S. Rubinsztajn, W. Fortuniak and J. Kurjata, Macromolecules, 2008, 41, 7352 CrossRef CAS.
- J. Kurjata, W. Fortuniak, S. Rubinsztajn and J. Chojnowski, Eur. Polym. J., 2009, 45, 3372 CrossRef CAS PubMed.
- M. A. Brook, J. B. Grande and F. Ganachaud, in Silicon Polymers, Advances in Polymer Science, ed. A. M. Muzafarov, Springer, Berlin, Heidelberg, 2011, vol. 235, pp. 161–183 Search PubMed.
- E. V. Egorova, N. G. Vasilenko, N. V. Demchenko, E. A. Tatarinova and A. M. Muzafarov, Dokl. Chem., 2009, 424, 15 CrossRef CAS.
- A. A. Bychkova, F. V. Soskov, A. I. Demchenko, P. A. Storozhenko and A. M. Muzafarov, Russ. Chem. Bull., 2011, 6, 2384 CrossRef PubMed.
- E. D. Niemeyer and F. V. Bright, J. Phys. Chem. B, 1998, 102, 1474 CrossRef CAS.
- K. Otake, S. E. Webber, P. Munk and K. P. Johnston, Langmuir, 1997, 13, 3047 CrossRef CAS.
- K. L. Toews, R. M. Shroll, C. M. Wai and N. G. Smart, Anal. Chem., 1995, 67, 4040 CrossRef CAS.
- C. Roosen, M. Ansorge-Schumacher, T. Mang, W. Leitner and L. Greiner, Green Chem., 2007, 9, 455 RSC.
- A. I. Cooper, J. Mater. Chem., 2000, 10, 207 RSC.
- P. Munshi and S. Bhaduri, Curr. Sci., 2009, 97, 63 CAS.
- GLC-curve corresponds to the sample in Table 1, entry 3. Dx means oligodimethylcyclosiloxane with x repeating units. Lx means linear oligodimethylsiloxanes with x repeating units and either 2 –OH; 1 –OH and 1 –OAlk, or 2 –OAlk end groups.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, laboratory device description, spectral, GPC data. See DOI: 10.1039/c4ra13619e |
| This journal is © The Royal Society of Chemistry 2015 |