Facile synthesis of Ag-doped ZnCdS nanocrystals and transformation into Ag-doped ZnCdSSe nanocrystals with Se treatment†
Ruosheng Zeng*ab,
Zhiguo Suna,
Sheng Caoc,
Rongan Shena,
Zuoji Liua,
Ying Xionga,
Jingtao Longa,
Jinju Zhengc,
Yunqiang Zhaoa,
Yayun Shena and
Dingsheng Wangb
aKey Lab for Functional Materials Chemistry of Guizhou Province, School of Chemistry and Materials Science, Guizhou Normal University, Guiyang 550001, P. R. China. E-mail: zengrsh@gznu.edu.cn
bDepartment of Chemistry, Tsinghua University, Beijing, 100084, P. R. China
cInstitute of Materials, Ningbo University of Technology, Ningbo 315016, P. R. China
First published on 21st November 2014
Abstract
High-quality, pure, and color-tunable Ag:ZnCdS quantum dots (d-dots) are prepared by optimization of the experimental conditions including Ag-doping concentration and Zn/Cd precursor ratio. Highly emissive Ag:ZnCdS/ZnS core/shell d-dots with photoluminescence quantum yield (PL QY) as high as 58% are constructed in situ by the growth of a ZnS shell around the crude Ag:ZnCdS solution, which is the highest PL QY reported to date for Ag-based semiconductor d-dots. The emission color of a Ag:ZnCdS d-dot can be tuned toward a larger red region by simple Se treatment at high temperature (220–260 °C). With Se treatment, Ag:ZnCdS alloyed d-dots are transformed into Ag:ZnCdSSe alloyed d-dots, and the corresponding optical changes of d-dots in this process are investigated systematically. This strategy provides a versatile approach for the preparation of other multinary semiconductor nanocrystals.
1. Introduction
Semiconductor nanocrystals (NCs) with excellent electronic and optical properties have been extensively explored in the past decades because of their potential applications in biomedical labeling, photovoltaic devices and display or lighting devices.1–8 Doped semiconductor quantum dots (d-dots) with a transition metal have recently been receiving a great deal of attention due to their high photoluminescence quantum yield (PL QY), long PL lifetime, zero self-absorption, tunable emission colors and thermally stable luminescence.9–24 Therefore, doping has been a promising strategy to manipulate new optical properties of semiconductor nanomaterials. Semiconductor NCs are typically doped with 3d transition-metal ions, such as Mn2+, Cu2+, Ag+ or Ni2+.9–11,25,26 An exciting feature of these d-dots is that the optical properties can be tuned through quantum confinement effects of the semiconductor host as well as through the type and concentration of transition-metal dopants. Apart from the largely developed binary intrinsic NCs providing short-lived bandedge excitonic emission, different doped alloyed NCs with various element compositions have also been explored as promising materials where the carrier recombination process is strong affected by the transition-metal impurity.27 As a result, they show broad impurity emission with a significant Stokes shift, larger emission window, and longer excited-state lifetime. All of these features enroll these NCs into a new family, which provides several new applications to nurture the charge carriers and implement them in light emitting, harvesting, and devices.Though the binary d-dots have been extensively studied in the past, the alloyed d-dots attract the limelight of recent researches due to their functional properties, which still remain mostly unexplored. It is well known that doping is very specific to the nature of the dopants and hosts. Hence, the crucial part of designing d-dots is the compatibility of the placement of dopants in the crystal lattice of the selected host NCs. Recently, several doped NCs have been developed which also show a bandgap-dependent tunable emission.27,28 Among doped tunable NC emitters, Ag-doped NCs possess a wide range of tunable emission due to the tunability of the host bandgap.9 As one of the most important II–VI semiconductors, the ZnCdS alloy can be a suitable host for the selected dopants and has already shown promise because its bandgap can be facilely tuned by the Zn/Cd ratio.29 In recent years, doping of ZnCdS nanostructures has inspired a great deal of interests.27,28 In particular, Ag, a group IB element, is usually a promising candidate for modifying semiconductor materials owing to its high exchange-current density and low activation energy. Thus, Ag-doping can provide a convenient way to regulate the physical properties of the intrinsic semiconductor.9,30 Though aqueous Ag-doped NCs have been achieved by several groups,31,32 oil-soluble Ag-doped NCs remain an important challenge because most Ag precursors are active and can be easily oxidized or decomposed by a conventional doping method. However, an unsolved challenge is that balancing the reactivity of multi-precursors for the successful synthesis of the targeted multiple compound NCs is difficult to achieve and the traditional synthetic method usually results in separate nucleation of other NCs.33 Therefore, it is desirable to develop a versatile method to obtain the multiple compound alloyed NCs.
In this paper, we report a synthetic method for high-quality, pure, color-tunable, and oil-soluble Ag:ZnCdS NCs and the changes in optical properties of these NCs with Se treatment. The entire process has been developed by the introduction of a Se precursor in the reaction system of the Ag:ZnCdS alloyed NCs, and a huge change in the optical properties of the NCs is observed. The absorption and PL emission of the d-dots shift to new positions with new feature at a much redder window, whereas, surprisingly, the size and crystal structure of the d-dots remain unchanged. Changes of the d-dot optical properties with Se-treatment temperature and different amounts of Se precursor have been studied in detail and are reported in this article. Our synthetic strategy will provide a versatile approach for the preparation of other multinary alloyed functional semiconductor NCs.
2. Experimental section
2.1 Chemicals
Zinc acetate (ZnAc2, 99.99%), cadmium oxide (CdO, 99.0%), silver nitrate (AgNO3, 99.8%), sulfur powder (S, 99.5%), and dodecanethiol (DDT, 98%) were purchased from Sinopharm Chemical Reagent Company. Oleic acid (OA, 90%), oleylamine (OLA, 80–90%), and 1-octadecene (ODE, 90%) were purchased from Alfa Aesar. Tributyl phosphine (TBP, 95%) was purchased from Aldrich. All chemicals were used without further purification.2.2 Preparation of stock solutions
The Zn stock solution (0.125 M) was obtained by dissolving 0.1098 g of ZnAc2 in 2 mL of OA, and 2 mL of ODE at 110 °C under Ar flow. The Cd stock solution (0.5 M) was obtained by dissolving 0.32 g of CdO in 5 mL of OA at 280 °C under Ar flow. The Ag stock solution (0.02 M) was obtained by dissolving 0.0068 g of AgNO3 in 2 mL of OLA under ultrasonics at 40 °C. The S stock solution (0.4 M) was obtained by dissolving 0.064 g of S powder in 5 mL of ODE at 80 °C. The Zn stock solution (0.5 M) for ZnS overcoating was prepared by dissolving 0.55 of ZnAc2 in 2 mL of OLA and 3 mL of ODE at 80–90 °C. The Se stock solution for Se treatment was obtained by dissolving 0.25 g of Se powder in 1.3 mL TBP at room temperature in a glove-box.2.3 Typical synthesis of Ag:ZnCdS and Ag:ZnCdS/ZnS NCs
0.96 mL of Zn stock solution, 0.16 mL of Cd stock solution, 0.4 mL of OA, and 3 mL of ODE were loaded in a three-necked flask. The reaction mixture was degassed by purging Ar for 15 min and the flask was then heated to 270 °C. At this temperature, the mixture of 0.05 mL of Ag stock solution and 0.4 mL of DDT was injected rapidly into the reaction flask. Immediately, 0.4 mL of S stock solution was injected into the reaction flask and kept at 250 °C for the growth of Ag:ZnCdS NCs. The growth process was monitored through UV-vis and PL spectra by taking aliquots. Finally, the reaction was allowed to cool down to room temperature, and the NCs were purified using methanol–hexanes extraction four times repeatedly.Overcoating of the ZnS shell was carried out on the crude Ag:ZnCdS core NCs after a growth period of 5 min. The Zn and S stock solutions were added dropwise into the reaction flask at intervals of 15 min for ZnS shell growth. The amounts of the injection solutions injected for each step were as follows: 0.1 mL of the Zn and S solutions for the first and second layer, 0.15 mL of the Zn solution and 0.1 mL of the S solution for the third layer, and only 0.2 mL of the Zn solution for the fourth layer.
2.4 Se treatment of Ag:ZnCdS NCs
The completely purified Ag:ZnCdS NCs (the amount of one pot in typical synthesis) dissolved in 1 mL of hexanes were mixed with 3 g of ODE. The flask was then pumped down with a mechanical pump at room temperature for 5 min and heated to 120 °C for another 20 min to remove the hexanes. Subsequently, the system was switched to an Ar flow, and 0.1 mL of TBP and 50 μL of Se–TBP stock solution were injected into the flask. The reaction mixture was further heated to 220 °C for Se treatment. Aliquots were taken at different intervals and injected into cold toluene to terminate growth of d-dots immediately for the optical measurements.2.5 Characterization
UV-vis and PL spectra were recorded on a Shimadzu UV-2450 and F-4600 spectrophotometer, respectively. The PL QY of d-dots was determined by comparing the integrated emission of the d-dots in solution with that of a fluorescent dye (rhodamine 6G in ethanol or rhodamine 101 in 0.01% HCl ethanol solution) with an identical optical density. Transmission electron microscope (TEM) images were recorded on a Hitachi model H-800 microscope operated at 200 kV. High-resolution transmission electron microscope (HRTEM) images were obtained using a JEOL-2010F microscope. Specimens were prepared by putting a drop of toluene solution containing d-dots (optical density ∼ 0.3) on a copper grid coated with thin carbon film. X-ray powder diffraction (XRD) patterns were obtained using a Bruker D8-Advance X-ray diffractometer with monochromatized Cu Kα radiation. The samples were ground and placed on a glass substrate for XRD characterization. Energy dispersive spectroscopy (EDS) was recorded to determine the composition of the obtained products. The specific Ag-doping concentration of samples after thorough purification was determined by inductively coupled plasma mass spectrometry (ICP-MS, Thermo ICP-MS XII). PL decays were recorded using a Horiba Jobin Yvon Fluromax-4p with a time-correlated single-photon-counting (TCSPC) spectrometer. A NanoLED solid-state pulsed diode emitting at 370 nm was utilized as the exciting source for PL decay measurement, and the time resolution of the TCSPC system was about 1 ns. The particle sizes were measured by dynamic light scattering (DLS) using a Malvern Zetasizer Nano-ZS90 (Malvern instruments, UK). All of the DLS measurements were performed at 25 °C and at a scattering angle of 90°. The volume of the samples used for size measurement was 0.2 mL. X-ray photoelectron spectroscopy (XPS) was performed on a Kratos Axis Ultra X-ray photoelectron spectrometer with an Al Kα source.3. Results and discussion
3.1 Influence of Ag dopant concentration on optical properties
It is well known that dopant concentration is a key factor for tailoring the optical properties of semiconductor NCs. To understand the effects of dopant concentration on PL properties of d-dots, a series of Ag:ZnCdS d-dot samples with different nominal Ag/(Zn + Cd) molar ratios (from 0.1% to 2%) were prepared while keeping the other experimental conditions unchanged. It should be noted that the real Ag doping concentration in Ag:ZnCdS NCs determined by ICP-MS is always lower than the nominal Ag/(Zn + Cd) molar ratios used in the synthesis (Table 1). The UV-vis absorption and PL emission spectra of the Ag:ZnCdS NCs prepared with different Ag/(Zn + Cd) molar ratios are shown in Fig. 1A, and the corresponding evolution of the PL intensity and peak position is summarized in Fig. 1B. With the increase in the amount of Ag stock solution, the PL intensity of Ag dopant emission increases. The PL QY of Ag dopant emission achieves a maximum (about 25%) when the Ag/(Zn + Cd) molar ratio is about 1%. If the amount of Ag precursor added is increased further, the normalized PL intensity of the Ag dopant emission decreases, which is probably due to more defects in the host lattice or stronger Ag–Ag interaction. Surprisingly, a significantly blue-shift of the Ag dopant PL emission is observed clearly with the increase of the nominal Ag ratio (Fig. 1B), which is different from the previous reports.20 Based on the obvious blue-shift of the adsorption bandgaps of samples (Fig. 1A), we can conclude that the bandgap energy of d-dots increases with the increase of the Ag-doping concentration in the host NCs. The ICP-MS results indicate that a greater Zn content proportion is incorporated into the host NCs with an increase of the Ag-doping concentration. A reasonable explanation is that the addition of Ag precursor affects the relative activity of the Zn and Cd precursor. With the increased bandgap of host NCs, the excited Ag dopant d-state shifts to a lower energy level and the dopant emission also exhibits a blue-shift because the dopant emission is attributed to the transition from the conduction band of the host to the level of the excited Ag d-state.9| a A: calculated from precursor ratios. B: real doping concentration from ICP-MS results. | ||||||
|---|---|---|---|---|---|---|
| A | 0.1 | 0.25 | 0.5 | 1.0 | 1.5 | 2.0 |
| B | 0.09 | 0.22 | 0.41 | 0.75 | 0.96 | 1.1 |
3.2 Tunable dopant emission based on host composition changes
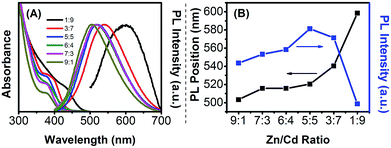
As described before, Ag dopant emission, like Cu emission, is derived from the recombination of the electron in the conduction band of host material and hole in the Ag d-state, which locates above the valence band of semiconductor host, and the valence hole transfers to this d-state after excitation.9 Therefore, Ag-based d-dots can be a potential color-tunable emitter dependent on the nature, size, and composition of the host NCs. Besides the particle size in binary NCs, compound alloyed NCs can also serve as a possible alternative to tune the bandgap of the host NCs through changing the host composition and accordingly tune the Ag dopant emission color. Compound ZnCdS-alloyed NCs are promising host materials for Ag-based NCs due to their wide tunable bandedge absorption window.Fig. 2 shows the UV-vis absorption and PL spectra and evolution of PL intensity and peak position of Ag:ZnCdS NCs under different Zn/Cd precursor ratios. The composition of host NCs is tuned through the changes in the Zn/Cd precursor ratio in the synthetic step. As shown in Fig. 2, the molar ratio of Zn/Cd precursors is varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 9
9 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the gross amount of Zn and Cd precursor kept at 0.2 mmol. It should be noted that no PL is observed in the resulting Ag:ZnCdS NCs when the Zn/Cd ratio is less than 1
1 and the gross amount of Zn and Cd precursor kept at 0.2 mmol. It should be noted that no PL is observed in the resulting Ag:ZnCdS NCs when the Zn/Cd ratio is less than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. Experimental results show that the first excitonic absorption peak of the intrinsic ZnCdS-alloyed NCs red-shifts systematically from 375 to 430 nm and the PL position of dopant emission red-shifts from 503 to 599 nm with a decrease of the nominal Zn/Cd precursor ratio from 9
9. Experimental results show that the first excitonic absorption peak of the intrinsic ZnCdS-alloyed NCs red-shifts systematically from 375 to 430 nm and the PL position of dopant emission red-shifts from 503 to 599 nm with a decrease of the nominal Zn/Cd precursor ratio from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. The PL QY first increases rapidly with decreasing Zn/Cd precursor and achieves the highest value of 25% at a Zn/Cd ratio of 5
9. The PL QY first increases rapidly with decreasing Zn/Cd precursor and achieves the highest value of 25% at a Zn/Cd ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and then decreases with further decreases the Zn/Cd precursor ratio. A remarkable red-shift of Ag dopant emission with the decrease of the Zn/Cd ratio is observed, which is due to the decreased bandgap of the alloyed NCs since the bandgap of CdS is much lower than that of ZnS.
5, and then decreases with further decreases the Zn/Cd precursor ratio. A remarkable red-shift of Ag dopant emission with the decrease of the Zn/Cd ratio is observed, which is due to the decreased bandgap of the alloyed NCs since the bandgap of CdS is much lower than that of ZnS.
 | ||
| Fig. 2 (A) Absorption and PL emission spectra, and (B) evolution of PL intensity and peak position of Ag:ZnCdS NCs under different Zn/Cd precursor ratios. | ||
3.3 Size, shape, and crystals structure of d-dots and influence of the ZnS shell on optical properties
It is well known that epitaxial growth of a wider bandgap shell on the bare core can further enhance the stability and PL QY of semiconductor d-dots because both electrons and holes are confined in the core and thus passivate the surface recombination sites of the original particles.12,34 In this work, to improve the PL QY and stability of the Ag:ZnCdS NCs, the core/shell Ag:ZnCdS/ZnS NCs were constructed in situ from the crude Ag:ZnCdS core reaction solution. Due to the existing excess sulfur in the crude reaction solution, less sulfur source was added during the process of depositing the ZnS shell. To avoid the formation of separate ZnS NCs, Zn precursor was added dropwise in portions (see details in the Experimental Section). The evolution of UV-vis absorption and PL spectra of Ag:ZnCdS/ZnS core/shell NCs starting from the bare Ag:ZnCdS NCs under a nominal Zn/Cd ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
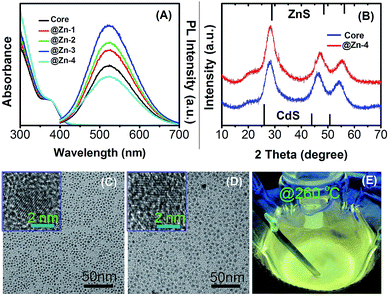
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 is shown in Fig. 3A. The results indicate that the growth of the ZnS shell enhances the PL QY of d-dots substantially. The substantial increase of absorption in the high energy part of the absorption spectra (roughly between 300 and 350 nm) reveals the gradual formation of the core/shell structure. After deposition of the ZnS shell (i.e., Zn-4 in Fig. 3A), the PL QY of Ag:ZnCdS/ZnS core/shell NCs can reach as high as 58% (Fig. 3E).
5 is shown in Fig. 3A. The results indicate that the growth of the ZnS shell enhances the PL QY of d-dots substantially. The substantial increase of absorption in the high energy part of the absorption spectra (roughly between 300 and 350 nm) reveals the gradual formation of the core/shell structure. After deposition of the ZnS shell (i.e., Zn-4 in Fig. 3A), the PL QY of Ag:ZnCdS/ZnS core/shell NCs can reach as high as 58% (Fig. 3E).
The XRD patterns, TEM and HRTEM images for the above representative Ag:ZnCdS/ZnS NCs starting from Ag:ZnCdS core NCs with a nominal Zn/Cd ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 are shown in Fig. 3B–D, respectively. The XRD patterns of Ag:ZnCdS/ZnS NCs preserve the zinc blende structure of the Ag:ZnCdS core NCs, but the diffraction peaks shift to the ZnS side further due to the increase of ZnS content, which also is an indication for the successful growth of ZnS shell.19 It should be pointed out that the broad diffractive peaks and relatively low peak height are typical for semiconductor NCs. As shown in Fig. 3C and D, a remarkable increase in particle size from about 3.6 nm to 5.0 nm can be observed upon shell growth. Considering a thickness of 0.62 nm for 1 monolayer (ML) of the ZnS shell, the thickness of the ZnS shell is estimated to be 2.3 MLs. The Ag:ZnCdS NCs after deposition of 2.3 ML ZnS are all nearly spherical in shape. The deposition of a ZnS shell not only dramatically decreases the surface defects and increases the PL QY, but also enhances the photostability of d-dots.
5 are shown in Fig. 3B–D, respectively. The XRD patterns of Ag:ZnCdS/ZnS NCs preserve the zinc blende structure of the Ag:ZnCdS core NCs, but the diffraction peaks shift to the ZnS side further due to the increase of ZnS content, which also is an indication for the successful growth of ZnS shell.19 It should be pointed out that the broad diffractive peaks and relatively low peak height are typical for semiconductor NCs. As shown in Fig. 3C and D, a remarkable increase in particle size from about 3.6 nm to 5.0 nm can be observed upon shell growth. Considering a thickness of 0.62 nm for 1 monolayer (ML) of the ZnS shell, the thickness of the ZnS shell is estimated to be 2.3 MLs. The Ag:ZnCdS NCs after deposition of 2.3 ML ZnS are all nearly spherical in shape. The deposition of a ZnS shell not only dramatically decreases the surface defects and increases the PL QY, but also enhances the photostability of d-dots.
3.4 Transformation of Ag:ZnCdS alloyed NCs toward Ag:ZnCdSSe alloyed NCs with Se treatment
As described in the previous paragraph, the emission color of Ag-based d-dots is tunable and dependent on the nature and composition of the host NCs. Balancing the reactivity of multi-precursors is found to be the key issue for the successful synthesis of the targeted compound alloyed d-dots. Alternatively, in this article we propose a novel method to obtain that Ag:ZnCdSSe alloyed d-dots by Se treatment of the bare and purified Ag:ZnCdS core d-dots. Fig. 4 shows a schematic presentation of Ag d-state within the host bandgap and the most plausible mechanism of the recombination process in the Ag:ZnCdSSe alloyed d-dots. As indicated in Fig. 4, the Ag d-state remains above the host valence band, the position of conduction band moves with variation of the Se percentage in Ag:ZnCdSSe d-dots, which determines the emission wavelength of the d-dots. | ||
| Fig. 4 Schematic diagram of the introduction of Ag dopant state within the host bandgap, and tunable color emissions by treating with Se. | ||
The temperature of Se treatment is a key factor in the formation rate of high quality semiconductor Ag:ZnCdSSe NCs. In order to determine the best temperature of Se treatment during the transformation, UV-vis absorption and PL emission spectra under different temperatures were recorded (Fig. 5). As seen from Fig. 5A, the reaction of Se treatment needs a relative high temperature, and PL emission exhibits nearly no shift with the addition of 50 μL Se stock solution at 180 °C, indicating that this transformation needs a relative high temperature and can be carried out only at temperatures over 180 °C. However, when the temperature is increased to 260 °C for the Se solution injection, the PL peak position shifts from 514 to 582 nm within 90 min, indicating an increased reaction rate at higher temperatures. As indicated in Fig. 5B, the results show that PL intensity exhibits a relative lower decrease at 180 °C than at 220 or 260 °C, which indicates that the ion exchange reaction of the Se precursor with host lattice is a direct factor of the resulting decrease of the PL QY of the d-dots. At the lower temperature, the ion exchange reaction is relative low or difficult to be carried, and thus the PL intensity only exhibits a slight decrease. However, the specific mechanism is still unclear and needs to be clarified by further investigations. Fig. 5C–E show the successive adsorption spectra of the Ag:ZnCdSSe d-dots obtained with the progress of the Se-treatment reaction. At higher reaction temperatures, for example, a significant red-shift from the adsorption spectra is observed at 260 °C, which is due to the lower bandgap of ZnCdSe than that of host ZnCdS (see details in the following text). The chemical composition of the resulting NCs was determined mainly by EDS (Fig. S1, ESI†).
Further, the composition of Ag:ZnCdSSe NCs is tuned via variation of the amount of Se precursor. In the experiments, the amount of Se precursor is varied from 25 to 75 μL with the other experimental conditions fixed (Fig. 6). As indicated in Fig. 6A, with an increased amount of Se precursor, the Ag:ZnCdSeS NCs exhibit a larger red-shift window. This indicates that the Se is incorporated into the host NCs successfully, and a larger ratio of Se in host NCs results in a decrease of transition energy from the conduction band of the host to the energy level of the d-state of the d-dots (see Fig. 4). Further, the PL QY of d-dots reduces systematically, which is probably due to the formation of surface state or lattice defects (Fig. 6B). The absorption spectra of d-dots with different Se precursor amounts are also shown in Fig. 6C–E. The absorption bandgap of d-dots exhibits consecutive red-shifts, and the red-shift is more distinct the more Se precursor is added into the reaction system. The red-shift can easily be attributed to the composition changes of the host NCs, since the size of the d-dots is unchanged (see Fig. S2 in the ESI†).
Fig. 7 presents UV-vis, PL, and PL excitation (PLE) spectra of Ag:ZnCdS and Ag:ZnCdSSe d-dots. PLE measurements indicate that PL in the broadband had similar excitation spectra, which means that this broadband was, indeed, not due to the size distribution of the d-dots. In principle, as the emission wavelength monitored in PLE shifts along the PL spectra of a d-dot sample from high energy to low energy, the PLE spectra also shift from high energy to low energy if a broad PL peak width is caused by size distribution. As shown in Fig. 7, the normalized PLE spectra are practically the same, which indicates that the emission at any wavelength is contributed to from the entire ensemble of d-dots in a given sample. The substantial energy gap between the absorption peak and PL peak in Fig. 7 further supports that the PL should be the targeted Ag dopant PL, and no separate nucleated particles, such as CdSe, are formed during the Se treatment of Ag:ZnCdS d-dots.
Unfortunately, there is confusion over the exact structural composition of these NCs. To the best of our knowledge, treatment with Se might lead to the formation of a core/shell or alloying by ion displacement. On the one hand, based on the fact that the Ag:ZnCdS cores are purified completely, no excess cation exists in the solution. Therefore, the possibility of a core/shell structure can be overruled in our case. On the other hand, the size of the d-dots from Ag:ZnCdS to Ag:ZnCdSSe remains nearly unchanged after Se treatment (Fig. S2†). From the DLS analysis, as shown in Fig. S3,† the hydrodynamic diameters were slightly larger than those obtained from TEM images. The polydispersity indices (PDIs) of the prepared Ag:ZnCdS and Ag:ZnCdSSe nanocrystal size distributions measured by DLS were 0.414 and 0.409, respectively. We noted that the size of the hydrodynamic diameters of Ag:ZnCdSSe d-dots was larger than the size of Ag:ZnCdS d-dots, which was probably due to the presence of the larger molecule weight capping ligand TBP during Se treatment. Therefore, we can conclude that alloyed NCs are formed because of the replacement of S by Se. These d-dots still retain the zinc-blende phase throughout the alloying process (Fig. S4, ESI†). We mention here that, although alloying with Se extends both the absorption and PL emission tenability window, the d-dots retain the typical original optical characteristics throughout the alloying process.
To further obtain structural information of the Ag:ZnCdS d-dots during Se treatment, XPS analysis was performed. Fig. 8 reveals the presence of Zn, Cd, S, and Ag in the Ag:ZnCdS d-dots and Zn, Cd, S, Se, and Ag in the Ag:ZnCdSSe d-dots, respectively. Typical peaks of Ag 3d at 368.1 and 374.3 eV were attributed to Ag(I) of the d-dots. This result is in agreement with previous reports.35 It should be noted that the peaks of Zn, Cd, and S shifted toward a lower energy, which indicated that Se was incorporated in the host and bond lengths and bond energies changed.
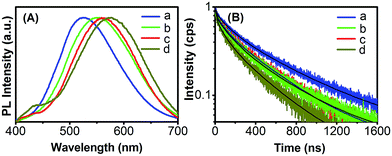
The PL lifetime measurements are valuable to investigate the mechanism of PL because different PL decay lifetimes may result from different recombination mechanisms.36 The decay kinetics of various Ag:ZnCdS samples treated with various amounts of Se precursor were measured and their corresponding PL emission spectra are shown in Fig. 9. For all four samples, the emissions detected at 516, 549, 560, and 573 nm for samples a, b, c, and d, respectively, match the composition-dependent PL peak position. In each case, the decay line is fitted with a biexponential function of I(t) = y0 + A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2
exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2), where τ1 and τ2 are the time constants and A1 and A2 are the normalized amplitudes of the components. The average lifetimes τav are determined by the expression τav = (A1τ12 + A2τ22)/(A1τ1 + A2τ2). The time constants of the decays are summarized in Table 2. From Table 2, the decay time constants of the two components are found to be on the scale of hundreds of ns and tens of ns, respectively.9,24 The slower ones can be attributed to the radiative transition of the electron in the host conduction band and hole in the Ag d-state, while the faster one is generally due to the contribution of nonradiative decay.37 The average lifetimes τav of the decays are 493.80, 401.21, 383.59, and 318.65 ns for samples a, b, c, and d, respectively, which are analogous with many previous reports.9,24 We find that the Se-treatment dependence of the PL lifetime is in excellent agreement with that of the PL QY, which can justify that the increase of the PL QY is from the passivation of the nonradiative channels. As shown in Table 2, our result shows that the lifetime of sample a is longer than the those of the other three Se-treated samples, which could be due to the formation of more nonradiative channels during Se treatment.
exp(−t/τ2), where τ1 and τ2 are the time constants and A1 and A2 are the normalized amplitudes of the components. The average lifetimes τav are determined by the expression τav = (A1τ12 + A2τ22)/(A1τ1 + A2τ2). The time constants of the decays are summarized in Table 2. From Table 2, the decay time constants of the two components are found to be on the scale of hundreds of ns and tens of ns, respectively.9,24 The slower ones can be attributed to the radiative transition of the electron in the host conduction band and hole in the Ag d-state, while the faster one is generally due to the contribution of nonradiative decay.37 The average lifetimes τav of the decays are 493.80, 401.21, 383.59, and 318.65 ns for samples a, b, c, and d, respectively, which are analogous with many previous reports.9,24 We find that the Se-treatment dependence of the PL lifetime is in excellent agreement with that of the PL QY, which can justify that the increase of the PL QY is from the passivation of the nonradiative channels. As shown in Table 2, our result shows that the lifetime of sample a is longer than the those of the other three Se-treated samples, which could be due to the formation of more nonradiative channels during Se treatment.
| Sample | Peak (nm) | A1 | τ1 (ns) | A2 | τ2 (ns) | τav (ns) |
|---|---|---|---|---|---|---|
| a | 573 | 0.33 | 69.54 | 0.66 | 522.06 | 493.80 |
| b | 560 | 0.33 | 58.66 | 0.63 | 425.92 | 401.21 |
| c | 534 | 0.36 | 62.61 | 0.60 | 412.80 | 383.59 |
| d | 531 | 0.46 | 43.75 | 0.56 | 347.11 | 318.65 |
3.5 Role of DDT in the synthesis
To the best of our knowledge, oil-soluble Ag-doped NCs are rarely reported because the most active Ag precursor can easily be decomposed by a conventional doping method. With AgNO3 dissolved in OLA as the Ag precursor, the formation of undoped ZnCdS NCs is found to be inevitable, even if the reaction temperature is low enough (<200 °C). This indicates that AgNO3 in OLA is too reactive and easily decomposed to metal Ag particles. Considering that Ag ions are very soft Lewis acids, we suspect that the hard Lewis base ligands (fatty acids) might not be good ligands to suppress the reactivity of the Ag ions. It is noted that thiols are used as the ligands in the synthesis of semiconductor NCs. Hence, we chose the soft Lewis base DDT as the ligand of Ag ions. Since DDT can make the Ag precursor much less reactive, the reaction temperature could be enhanced to about 260–270 °C and high-quality Ag:ZnCdS d-dots could be obtained in our experiments.4. Conclusion
In summary, high-quality, pure, color-tunable Ag:ZnCdS d-dots are successfully synthesized by control of the Ag-doping concentration and Zn/Cd ratio. Through epitaxial growth of the ZnS shell as the passivation layer, highly luminescent Ag:ZnCdS/ZnS core/shell d-dots with PL QY as high as 58% are constructed in situ around the crude Ag:ZnCdS core NCs. Further, the Ag:ZnCdS alloyed NCs can be transformed into Ag:ZnCdSSe alloyed NCs with Se treatment, though the d-dots retain the typical original optical characteristics throughout the alloying process, and the shape, size and crystal phase of the d-dots remain unchanged. This strategy provides a versatile approach for the preparation of other multinary alloyed NCs.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21101038, 21461006, 51472053, and 61106066), Project for Returnee (no. [2012]01), Project of International Cooperation in Science and Technology (qian ke he G zi [2014]7008), United Foundation of Guizhou Science and Technology Department-Guizhou Normal University (LKS no. [2012]18), and the Undergraduate Students' Innovation and Entrepreneurship Training Plan of Guizhou province (no. 201410663011). The authors thank Prof. Jialong Zhao for help and valuable discussion, and Dr Ping Gong for the measurement of DLS experiments.References
- Z. A. Peng and X. G. Peng, J. Am. Chem. Soc., 2001, 123, 183–184 CrossRef CAS.
- Z. X. Pan, I. Mora-Seró, Q. Shen, H. Zhang, Y. Li, K. Zhao, J. Wang, X. H. Zhong and J. Bisquert, J. Am. Chem. Soc., 2014, 136, 9203–9210 CrossRef CAS PubMed.
- X. Yuan, J. Hua, R. S. Zeng, D. H. Zhu, W. Y. Ji, P. T. Jing, X. D. Meng, J. L. Zhao and H. B. Li, Nanotechnology, 2014, 25, 435202 CrossRef PubMed.
- R. S. Zeng, T. T. Zhang, J. C. Liu, S. Hu, Q. Wan, X. M. Liu, Z. W. Peng and B. S. Zou, Nanotechnology, 2009, 20, 095102 CrossRef PubMed.
- C. M. Tyrakowski and P. T. Snee, Phys. Chem. Chem. Phys., 2014, 16, 837–855 RSC.
- Y. F. Ma, Y. Li and X. H. Zhong, RSC Adv., 2014, 4, 45473 CAS.
- Z. T. Deng, L. Cao, F. Q. Tang and B. S. Zou, J. Phys. Chem. B, 2005, 109, 16671–16675 CrossRef CAS PubMed.
- W. Chung, H. Jung, C. H. Lee and S. H. Kim, J. Mater. Chem. C, 2014, 2, 4227–4232 RSC.
- S. Jana, G. Manna, B. B. Srivastava and N. Pradhan, Small, 2013, 9, 3753–3758 CrossRef CAS PubMed.
- N. S. Karan, S. Sarkar, D. D. Sarma, P. Kundu, N. Ravishankar and N. Pradhan, J. Am. Chem. Soc., 2011, 133, 1666–1669 CrossRef CAS PubMed.
- R. S. Zeng, M. Rutherford, R. G. Xie, B. S. Zou and X. G. Peng, Chem. Mater., 2010, 22, 2107–2113 CrossRef CAS.
- R. S. Zeng, T. T. Zhang, G. Z. Dai and B. S. Zou, J. Phys. Chem. C, 2011, 115, 3005–3010 CAS.
- N. Pradhan, D. M. Battaglia, Y. C. Liu and X. G. Peng, Nano Lett., 2007, 7, 312–317 CrossRef CAS PubMed.
- N. Pradhan and X. G. Peng, J. Am. Chem. Soc., 2005, 129, 3339–3347 CrossRef PubMed.
- D. J. Norris, N. Yao, F. T. Charnock and T. A. Kennedy, Nano Lett., 2001, 1, 3–7 CrossRef CAS.
- N. Pradhan, D. Goorskey, J. Thessing and X. G. Peng, J. Am. Chem. Soc., 2005, 127, 17586–17587 CrossRef CAS PubMed.
- R. N. Bhargava, D. Gallagher, X. Hong and A. Nurmikko, Phys. Rev. Lett., 1994, 72, 416–419 CrossRef CAS.
- R. Buonsanti and D. J. Milliron, Chem. Mater., 2013, 25, 1305–1317 CrossRef CAS.
- R. S. Zeng, R. A. Shen, Y. Q. Zhao, Z. G. Sun, X. S. Li, J. J. Zheng, S. Cao and B. S. Zou, CrystEngComm, 2014, 16, 3414 RSC.
- R. S. Zeng, R. A. Shen, Y. Q. Zhao, X. S. Li, Z. G. Sun and Y. Y. Shen, Nanotechnology, 2014, 25, 135602 CrossRef PubMed.
- R. Bose, S. Jana, G. Manna, S. Chakraborty and N. Pradhan, J. Phys. Chem. C, 2013, 117, 15835–15841 CAS.
- J. X. Sun, G. Chen, Y. J. Feng and Y. Wang, RSC Adv., 2014, 4, 44466–44471 RSC.
- X. M. Xu, L. Y. Zhou, Q. Zhang and R. F. Wang, RSC Adv., 2013, 3, 24593–24596 RSC.
- X. Yuan, J. J. Zheng, R. S. Zeng, P. T. Jing, W. Y. Ji, J. L. Zhao, W. Y. Yang and H. B. Li, Nanoscale, 2014, 6, 300–307 RSC.
- B. T. Luong, E. Hyeong, S. Yoon, J. Choi and N. Kim, RSC Adv., 2013, 3, 23395–23401 RSC.
- C. L. Wang, S. H. Xu, Y. J. Shao, Z. Y. Wang, Q. Y. Xu and Y. P. Cui, J. Mater. Chem. C, 2014, 2, 5111–5115 RSC.
- B. B. Srivastava, S. Jana and N. Pradhan, J. Am. Chem. Soc., 2011, 133, 1007–1015 CrossRef CAS PubMed.
- W. J. Zhang, X. G. Zhou and X. H. Zhong, J. Phys. Chem. C, 2012, 51, 3579–3587 CAS.
- X. H. Zhong, Y. Y. Feng, W. Knoll and M. Y. Han, J. Am. Chem. Soc., 2003, 125, 13559–13563 CrossRef CAS PubMed.
- B.-J. Huang, F. Li, C.-W. Zhang, P. Li and P.-J. Wang, RSC Adv., 2014, 4, 41819–41824 RSC.
- Y. Y. Chen, L. J. Huang, S. J. Li and D. C. Pan, J. Mater. Chem. C, 2013, 1, 751–756 RSC.
- L. Lin, Y. Q. Wen, Y. X. Liang, N. Zhang and D. Xiao, Anal. Methods, 2013, 5, 457–464 RSC.
- R. G. Xie, M. Rutherford and X. G. Peng, J. Am. Chem. Soc., 2009, 131, 5691–5697 CrossRef CAS PubMed.
- A. K. Parchur, A. A. Ansari, B. P. Singh, T. N. Hasan, N. A. Syed, S. B. Rai and R. S. Ningthoujam, Integr. Biol., 2014, 6, 53–64 RSC.
- L. Q. Ye, J. Y. Liu, C. Q. Gong, L. H. Tian, T. Y. Peng and L. Zan, ACS Catal., 2012, 2, 1677–1683 CrossRef CAS.
- A. K. Parchur, A. I. Prasad, A. A. Ansari, S. B. Rai and R. S. Ningthoujam, Dalton Trans., 2012, 11032–11045 RSC.
- S. Cao, J. J. Zheng, J. L. Zhao, L. Wang, F. M. Gao, G. D. Wei, R. S. Zeng, L. H. Tian and W. Y. Yang, J. Mater. Chem. C, 2013, 1, 2540–2547 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11064a |
| This journal is © The Royal Society of Chemistry 2015 |