Efficient green-red piezofluorochromism of bisanthracene-modified dibenzofulvene†
Abstract
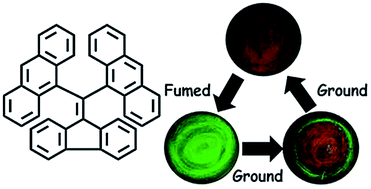
A general approach for the synthesis of dibenzofulvene-based derivatives is demonstrated. The bisanthracene modified dibenzofulvene (F-DAn) exhibits efficient piezofluorochromic properties with the fluorescence reversibly altered between green (536 nm) and red (620 nm) under external stimuli.

 Please wait while we load your content...
Please wait while we load your content...