Control of swelling–deswelling behavior of a self-oscillating gel by designing the chemical structure†
Tsukuru Masudaa,
Ayako Terasakia,
Aya Mizutani Akimotoa,
Kenichi Nagaseb,
Teruo Okanob and
Ryo Yoshida*a
aDepartment of Materials Engineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: ryo@cross.t.u-tokyo.ac.jp; Fax: +81-3-5841-7112; Tel: +81-3-5841-7112
bInstitute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical Univsersity (TWIns), 8-1 Kawada-cho, Shinjuku-ku, Tokyo 162-8666, Japan
First published on 12th December 2014
Abstract
We have developed a novel “self-oscillating” gel that exhibits an autonomous mechanical oscillation without any external stimuli. Here the ternary self-oscillating polymer composed of N-isopropylacrylamide and N-(3-aminopropyl)methacrylamide and Ru(bpy)3 was newly synthesized by atom transfer radical polymerization (ATRP) and the gel was prepared. For the self-oscillating polymers and gels with various compositions, their phase transition and self-oscillating behaviors were investigated considering the potential for biomedical application. It was demonstrated that the swelling–deswelling behavior of the self-oscillating gels can be controlled by changing the composition ratio of the free amino group present in the polymer and the conjugated Ru(bpy)3 moieties. Therefore the hydrophilic/hydrophobic balance is controllable and adjustable by this composition ratio.
Introduction
Stimuli-responsive polymer gels which respond to external stimuli such as temperature, pH, light, and chemicals have attracted much attention as smart materials. They have been investigated for several kinds of applications including artificial muscles, biosensors, microfluidics, drug delivery systems, cell culture materials, etc.1–9 In contrast, we have developed a novel “self-oscillating” gel that exhibits an autonomous mechanical swelling–deswelling oscillation without any on–off switching of external stimuli.10–12 The basic chemical structure of the self-oscillating polymer is a copolymer of N-isopropylacrylamide (NIPAAm) and Ru(bpy)3 as a catalyst for the Belousov–Zhabotinsky (BZ) reaction, which is a chemical oscillating reaction accompanying a spontaneous redox oscillation of the catalyst. Redox changes of Ru(bpy)3 moiety induces the hydration/dehydration changes of the polymer chains because the hydrophilicity of the polymer changes with an alternation in the valency and the LCST shifts to higher temperature in the oxidized state.13 When the polymer gel is immersed in an aqueous solution containing all the substrates of the BZ reaction except for the catalyst, the reaction occurs in the gel phase. As a result, the gel undergoes spontaneous and cyclic swelling–deswelling changes with the redox changes of Ru(bpy)3. Recently, there are many studies related to the self-oscillating gels,14 including theoretical simulations on chemomechanical behaviors.15,16Since the first report in 1996,10 we have systematically studied the self-oscillating polymers and the gels as well as their applications to biomimetic smart materials,11,12 including biomimetic actuators, autonomous transport systems utilizing peristaltic motion of the gel surface or the tubular gel, functional fluids showing viscosity oscillation, etc.17–19 In these materials design, polymer architecture is important to control the self-oscillating behavior. We have already shown several strategies to improve the dynamic properties of the polymers and gels by designing chemical and physical network structures such as introducing other components,20 microgel-aggregated structure,21 comb-type network structure.22 Further, well-defined diblock copolymers composed of hydrophilic and self-oscillating segments were prepared by reversible addition fragmentation chain transfer (RAFT) copolymerization, and it was demonstrated that the block copolymers undergo unimer/micelle23 or unimer/vesicle24 oscillation. Studies on these multiblock polymer systems are still in progress.
In addition, recently we reported the preparation of precisely controlled self-oscillating polymer chains and polymer brushes grafted on glass substrate by atom transfer radical polymerization (ATRP) and surface-initiated ATRP (SI-ATRP), respectively.25 A copolymer of NIPAAm and N-(3-aminopropyl)methacrylamide (NAPMAm) was prepared as the first step, and then Ru(bpy)3 having a succinimidyl group was conjugated to the polymer through the amino group of NAPMAm as the second step. By changing the composition of NIPAAm, NAPMAm, and Ru(bpy)3, the property of the self-oscillating polymer could be controlled more precisely. In particular, it is possible to leave unreacted amino groups in the polymer chain by adding less Ru(bpy)3 in the second step. These remaining amino groups would affect the properties of the polymers, and the hydrophilic/hydrophobic balance in the poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) can be controlled. The effects of Ru(bpy)3 amount and the hydrophilic/hydrophobic balance on the self-oscillating behavior of the linear polymer can be applied to the gels. As an one step toward future applications to biomaterials, these precise studies would offer a valuable guideline to cause effective self-oscillation around human body temperature.
In this study, the ternary self-oscillating polymer and the gel composed of NIPAAm, NAPMAm, and Ru(bpy)3 with various composition were prepared, and their phase transition and self-oscillating behaviors were evaluated and compared with typical self-oscillating poly(NIPAAm-co-Ru(bpy)3) gel.
Experimental
Materials
N-Isopropylacrylamide (NIPAAm) was kindly provided by KJ Chemicals (Tokyo, Japan) and purified by recrystallization in toluene/hexane. N-3-(Aminopropyl) methacrylamide (NAPMAm) was purchased from Polysciences (Warrington, PA, USA) and used as received. Bis(2,2′-bipyridine) (1-(4′-methyl-2,2′-bipyridine-4-carbonyloxy)-2,5-pyrrolidinedione) ruthenium(II) bis(hexafluorophosphate) (abbreviated as Ru(bpy)3–NHS) was synthesized according to previous report.26 Tris(2-(N,N-dimethylamino)ethyl)amine (Me6TREN) was purchased from Aldrich. Ethyl 2-bromoisobutyrate, copper(I) bromide (CuBr), dehydrated dimethyl sulfoxide (DMSO) were purchased from Wako Pure Chemical Industries (Osaka, Japan). N,N′-Methylenebisacrylamide (MBAAm) was purchased from Acros Organics (Pittsburg, PA, USA). Tetramethylenediamine (TEMED) was purchased from Wako Pure Chemical Industries (Osaka, Japan). Ammonium persulfate (APS) was purchased from Kanto Chemical (Tokyo, Japan). Sodium carbonate and sodium bromate (NaBrO3) was purchased from Kanto Chemical (Tokyo, Japan). 1 M HNO3 aqueous solution and malonic acid (MA) were purchased from Wako Pure Chemical Industries (Osaka, Japan).Preparation of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm)
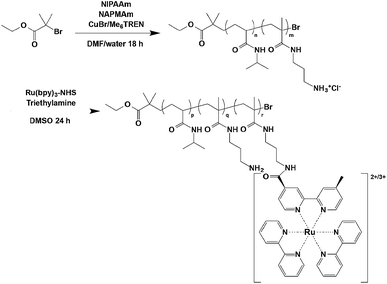
Poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) was prepared by ATRP as previously reported (Scheme 1).25 Briefly, NIPAAm (50.4 mmol) and NAPMAm (5.6 mmol) (the monomer composition: NAPMAm 10 mol%) were dissolved in a water/DMF = 1/1 (v/v) solution and the mixture was deoxygenated. CuBr (0.56 mmol), Me6TREN (0.56 mmol) and ethyl 2-bromoisobutyrate (0.56 mmol) were then added to the solution. The ATRP reaction was carried out for 18 h at 25 °C. The reaction solution after polymerization was dialyzed against 0.1 M Na2CO3 aqueous solution and distilled water, and the polymer was recovered by freeze-drying. The obtained polymer was dissolved in DMSO solution of 70 mM Ru(bpy)3–NHS containing triethylamine, and stirred for 24 h at 25 °C. The polymer solution was dialyzed against water and the polymer was recovered by freeze-drying.Characterization of the polymers
Number-average molecular weights and polydispersity index (PDI) values of the copolymers were determined by a gel permeation chromatography (GPC) (Tosoh, Tokyo), using DMF containing 50 mM LiCl as a mobile phase. A calibration curve was obtained using poly(ethylene glycol) standards. NAPMAm content in the copolymers was determined by 1H NMR (JOEL, JNM-LA400WB) using D2O as a solvent.Preparation of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) gel
NIPAAm, NAPMAm, MBAAm, and TEMED were dissolved in distilled water (the monomer composition of NAPMAm: 2.5, 5, and 10 mol%). After the pre-gel solution was cooled in iced water under a nitrogen atmosphere for 30 min, the initiator APS was added. The glass capillaries with inner diameter 500 μm were put in the ampoule and radical copolymerization was conducted at 4 °C. After gelation, all of the cylindrical gels were taken out of the glass capillaries by immersing 0.1 M Na2CO3 aqueous solution to make the gels deswollen. The prepared gels were thoroughly washed with distilled water to remove unreacted monomers for two days. The gels were immersed in DMSO to exchange solvent. After that, the gels were immersed in DMSO solution of 70 mM Ru(bpy)3–NHS containing triethylamine for 24 h to conjugate Ru(bpy)3 to amino group of the gels. Then, the gels were thoroughly washed with DMSO to remove unreacted reagents. Prepared gels were preserved in distilled water. All samples are referred as PNNR-x-(y%) where x and y represent the fed composition of NAPMAm and the fed amount of Ru(bpy)3–NHS (70 mM corresponds 100%), respectively.Measurements of LCST behaviors of the polymer solution
The optical transmittance of the poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) solution was measured by using a UV-vis spectrophotometer (UV-2500 PC, Shimazu, Japan). Transmittance changes at 583.5 nm as a function of temperature were measured in the oxidized Ru(III) and reduced Ru(II) states in 1 M HNO3 solutions containing 3 mM Ce(SO4)2 and 3 mM Ce2(SO4)3, respectively. The onset of transmittance change was used in estimating the lower critical solution temperature (LCST). Here, for convenience, LCST of the self-oscillating polymer was defined as the temperature when the transmittance of the solution decreased by 10% from the initial state.Measurements of self-oscillating behaviors of the polymer solutions
0.05 wt% poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) aqueous solution containing MA (0.1 M), NaBrO3 (0.15 M), and HNO3 (0.81 M) was prepared and the time course of transmittance changes at 15, 20, 25, 30, 35 and 37 °C was measured.Measurements of equilibrium swelling ratio of the gel
The gel samples were equilibrated in the solutions: 5 mM Ce(SO4)2 and 894 mM HNO3 for the oxidized Ru(III) state; 5 mM Ce2(SO4)3 and 894 mM HNO3 for the reduced Ru(II) state. The diameters of the cylindrical gel samples were measured by using an optical microscope (Leica, MZ16). The swelling ratio was defined as the diameter of the gel normalized by the inner diameter of the glass capillary (d0 = 500 μm) used in the gelation process.Measurements of self-oscillating behaviors of the gel
The cylindrical gels were immersed in the catalyst free-BZ reaction solution containing HNO3, NaBrO3, and MA at 20 °C and 37 °C. Oscillating behaviors of the gels were recorded by a digital video recorder through a CCD camera (Toshiba Teli, CS5270B) attached to a microscope (MZ16, Leica).Results and discussions
Characterization of the linear polymers
Ternary self-oscillating polymer, poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm), was prepared by ATRP as mentioned in the experimental section. The molecular weight (Mn) and polydispersity index (Mw/Mn) determined by GPC were 1.79 × 104 and 1.13, respectively. The composition ratio of poly(NIPAAm-co-NAPMAm) synthesized firstly was determined to be NIPAAm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NAPMAm = 90.9
NAPMAm = 90.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.1 by 1H-NMR. To estimate the introducing ratio of Ru(bpy)3 in the polymer chain quantitatively, the absorbance at 460 nm of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) solution was measured by using a UV-vis spectrophotometer and the ratio was calculated by using a calibration curve (Fig. S1†). The conjugation ratio of Ru(bpy)3–NHS to poly(NIPAAm-co-NAPMAm) was calculated to be 71% (see Fig. S1 in ESI†). Thus, the composition ratio of the ternary polymer was determined to be NIPAAm
9.1 by 1H-NMR. To estimate the introducing ratio of Ru(bpy)3 in the polymer chain quantitatively, the absorbance at 460 nm of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) solution was measured by using a UV-vis spectrophotometer and the ratio was calculated by using a calibration curve (Fig. S1†). The conjugation ratio of Ru(bpy)3–NHS to poly(NIPAAm-co-NAPMAm) was calculated to be 71% (see Fig. S1 in ESI†). Thus, the composition ratio of the ternary polymer was determined to be NIPAAm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NAPMAm
NAPMAm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ru(bpy)3NAPMAm = 90.9
Ru(bpy)3NAPMAm = 90.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5. The Ru(bpy)3 ratio in the ternary self-oscillating polymer prepared here was higher than that of the self-oscillating polymer (poly(NIPAAm-co-Ru(bpy)3)) prepared in the previous studies.13 The lower composition ratio of Ru(bpy)3 in the poly(NIPAAm-co-Ru(bpy)3 was attributed to low solubility of Ru(bpy)3 vinyl monomer in the solvent used for the one-step random copolymerization. However, by conjugating Ru(bpy)3–NHS to amino group of poly(NIPAAm-co-NAPMAm), the self-oscillating polymer with higher ratio of Ru(bpy)3 composition was successfully prepared.
6.5. The Ru(bpy)3 ratio in the ternary self-oscillating polymer prepared here was higher than that of the self-oscillating polymer (poly(NIPAAm-co-Ru(bpy)3)) prepared in the previous studies.13 The lower composition ratio of Ru(bpy)3 in the poly(NIPAAm-co-Ru(bpy)3 was attributed to low solubility of Ru(bpy)3 vinyl monomer in the solvent used for the one-step random copolymerization. However, by conjugating Ru(bpy)3–NHS to amino group of poly(NIPAAm-co-NAPMAm), the self-oscillating polymer with higher ratio of Ru(bpy)3 composition was successfully prepared.
LCST behaviors of the linear polymer
The phase transition behavior of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) solution was investigated in both of the reduced and oxidized states. Fig. 1 shows the temperature dependence of the optical transmittance of the polymer solution in the reduced Ru(II) state and the oxidized Ru(III) state at 583.5 nm, which is an isosbestic point of the Ru(bpy)3 moiety (see Fig. S2 in ESI†). Due to the characteristics of the thermo-responsive NIPAAm component, the transmittance of the polymer solutions in the both redox states suddenly decreased as temperature increased. The critical temperature is called a lower critical solution temperature (LCST). When the Ru(bpy)3 moiety was kept in the oxidized state, the LCST shifted higher than that in the reduced state. This is because the hydrophilicity of the polymer increased with an increase in valency of the conjugated Ru(bpy)3.13 | ||
| Fig. 1 Temperature dependence of optical transmittance for poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) solutions under the different conditions of the reduced Ru(II) state and oxidized Ru(III) state. | ||
The LCST of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) in the oxidized state was ca. 60 °C and that in the reduced state was ca. 10 °C. The difference of the two LCSTs was approximately 50 °C, which was characteristic in contrast to poly(NIPAAm-co-Ru(bpy)3). In our previous study, the LCST of poly(NIPAAm-co-Ru(bpy)3) in the oxidized and reduced states was 28 °C and 20 °C, respectively,13 and the difference was approximately 8 °C. These results may be simply explained by the larger amount of Ru(bpy)3 conjugated to poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) compared to that in poly(NIPAAm-co-Ru(bpy)3). However, it also suggests that the unreacted NAPMAm with free amino group contributes to the increase in the LCST difference. In the previous work, it was revealed that the LCST of poly(NIPAAm-co-Ru(bpy)3) in the oxidized state is only 1 °C higher than 32 °C (i.e., the LCST of NIPAAm homopolymer (PNIPAAm)) while that in the reduced state is strongly dependent on Ru(bpy)3 content. This means that hydrophobicity of Ru(bpy)3 originated from bipyridine ligand is still more dominant than the change in charge number. Therefore, it is effective to increase total hydrophilicity of polymer chain by introducing amino group. The LCST of poly(NIPAAm-co-NAPMAm) was ca. 50 °C (see Fig. S3 in ESI†), which is higher than the LCST of PNIPAAm. For the higher original LCST of poly(NIPAAm-co-NAPMAm), by introducing Ru(bpy)3, LCST of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) becomes much higher in the oxidized state and much lower in the reduced state. As a result, since the difference of the LCSTs in the two redox states becomes larger, it is expected that oscillation can be caused in wider temperature range by using poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm), compared to poly(NIPAAm-co-Ru(bpy)3).
Self-oscillating behavior of the polymer at different temperatures
Fig. 2 shows the oscillating profiles of the optical transmittance for the poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) solution containing the BZ reaction substrates (HNO3, NaBrO3, MA) at several temperatures. It should be noticed that the transmittance changes indicate solubility changes of the polymer because the wavelength at an isosbestic point is used. Soluble–insoluble oscillation successfully occurred in wider temperature range from 15 °C to 37 °C than the range for poly(NIPAAm-co-Ru(bpy)3). This is owing to increase of solubility of the self-oscillating polymer in the oxidized state at higher temperatures. As temperature increased, the oscillation period became shorter as typically observed in the BZ reaction (see Fig. S4 in ESI†).Temperature dependence of equilibrium swelling ratio for the gels
Then, the composition of the poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) gels was modified and their swelling–deswelling behaviors were compared. First, the effect of NAPMAm composition of polymer gels was investigated. Poly(NIPAAm-co-NAPMAm) gels with various composition were prepared by modulating the fed composition of NAPMAm (2.5 mol%, 5 mol%, and 10 mol%). Next, an excess amount of Ru(bpy)3–NHS was fed in conjugation to amino group of NAPMAm. That is, all the amino groups were substituted Ru(bpy)3 moieties. Fig. 3 shows the equilibrium swelling ratio of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) gels with various NAPMAm compositions. As NAPMAm composition increased, amount of Ru(bpy)3 conjugated to the gels increased accompanying the increase in amino group. As a result, differences of the swelling ratios between two redox states became larger by increasing NAPMAm composition. In the case of PNNR-10-(100%), the difference of the swelling ratio between the two states was approximately 30% around 37 °C. | ||
| Fig. 3 The equilibrium swelling ratio of (a) PNNR2.5-(100%), (b) PNNR5-(100%), and (c) PNNR10-(100%) in the reduced and oxidized states as a function of temperature. | ||
These results suggested that the kinetics of the diameter change of the gel in respond to the redox change would be improved, owing to the larger differences of the swelling ratios between two redox states. Actually, the swelling and deswelling kinetics of swelling process and deswelling process of gels were improved by increasing the amount of Ru(bpy)3 compared to the typical poly(NIPAAm-co-Ru(bpy)3) gel (see Fig. S5 and Table S1 in ESI†).22 The amount of Ru(bpy)3 introduced to the gels effected on not only the equilibrium swelling ratio but also the swelling–deswelling kinetics in response to the redox change of Ru(bpy)3. Thus, increase of the amplitude of swelling–deswelling oscillation was expected.
Self-oscillating behavior of the gels
Fig. 4 shows oscillating profile of PNNR-5-(100%) and PNNR-10-(100%) in a catalyst-free BZ reaction solution at 20 °C (“amplitude” is defined as a difference between the maximum and the minimum values of L/L0. Here L0 is the minimum length of the gel during the oscillating changes.). At the earlier stage, in both cases Fig. 4(a) and (b), it seems that the oscillation is stable but the swelling–deswelling amplitude is smaller compared with that at the later stage. Since the swelling or deswelling rate of the gel is typically slower than the rate of the chemical reduction or oxidation, the gel cannot follow the redox changes and cannot swell or shrink completely when oscillation period is short. At the later stage, however, the oscillation period increases probably because the substrates are gradually consumed as the BZ reaction proceeds (Fig. S6 in ESI†). By increasing period, the swelling–deswelling amplitude increases because the duration for swelling or deswelling is elongated. As a result of such compensation between reaction and swelling kinetics, oscillating profiles showing an increase in both period and amplitude at later stage might be observed. Although detailed mechanisms are still under investigation, the amplitude of the later stage was larger than the conventional poly(NIPAAm-co-Ru(bpy)3) gel.22Swelling–deswelling oscillation of PNNR-10-(100%) at 37 °C was not observed because the gel was shrunken in the reduced state and the reaction substrates did not diffuse into the polymer gel. In order to realize self-oscillation at the physiological temperature, it was necessary to decrease hydrophobicity of the polymer gel in the reduced state. For this purpose, hydrophilic/hydrophobic balance of the polymer was adjusted by changing the amount of conjugated Ru(bpy)3 and keeping a 10 mol% NAPMAm. The composition of the crosslinker (MBAAm) was also decreased from 4 mol% to 2 mol%. Fig. 5 shows the equilibrium swelling ratio of poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) gels with various Ru(bpy)3 compositions. As the amount of Ru(bpy)3 decreased, the difference in the swelling ratios between the redox states decreased while the swelling ratio in the reduced state increased. The inflection points around 27 °C may be due to the transition behavior of the gels, which is typically observed in the previous studies.27 For these gels, swelling–deswelling oscillation was observed at 37 °C. Especially, in the case of PNNR-10-(12.5%), the oscillation amplitude was the largest (Fig. 6). Although the amplitude (ca. 3%) is comparable with that for the conventional gels,22 considering that oscillating period becomes shorter due to increasing temperature, the gel has enough swelling–deswelling response. The response could be improved by designing the physical structure of the gel such as introducing porous structure or comb-type network structure, as we have already reported.21,22 Here it was demonstrated that extension or adjustment of the temperature range is possible by controlling the amount of unreacted amino groups within the polymer.
 | ||
| Fig. 5 The equilibrium swelling ratio of (a) PNNR10-(25%), (b) PNNR10-(12.5%), and (c) PNNR10-(10%) in the reduced and oxidized states as a function of temperature. | ||
Conclusions
In this study, the ternary self-oscillating polymer, poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm), was synthesized by ATRP and the gels were prepared. The difference in LCSTs between the oxidized and reduced states of the ternary polymer became much larger than that of poly(NIPAAm-co-Ru(bpy)3) because larger amount of Ru(bpy)3 can be conjugated and the remaining amino group increases total hydrophilicity of the polymer. Soluble–insoluble oscillation of the polymer chain at a wider temperature range was demonstrated. Based on this knowledge, poly(NIPAAm-co-NAPMAm-co-Ru(bpy)3NAPMAm) gel was prepared and the effect of composition on the self-oscillating behavior was investigated. Differences of the swelling ratios between two redox states became larger by increasing NAPMAm composition, under the condition that all the amino groups were substituted Ru(bpy)3 moieties. When the NAPMAm composition was 10 mol%, oscillating profiles showing an increase in both period and amplitude were observed at 20 °C. The swelling–deswelling oscillation with a comparable amplitude around physiological temperature (37 °C) was also achieved by controlling the amount of Ru(bpy)3 conjugated to the gel, which expands potential application of the gels to biomedical fields. It was demonstrated that swelling–deswelling behaviors of the self-oscillating gels can be controlled by changing the composition ratio of amino group remaining in the polymer and conjugated Ru(bpy)3 moieties to adjust hydrophilic/hydrophobic balance.Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research to R.Y. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant no. 22245037) and Research Fellowships to T.M. from the Japan Society for the Promotion of Science for Young Scientists (no. 269992). We acknowledge Dr Zulma A. Jimenez for helpful suggestions.Notes and references
- T. Miyata, Supra-molecular Design for Biological Applications, ed. Yui N., CRC, Boca Raton, 2002, pp. 191–225 Search PubMed.
- F. Liu and M. W. Urban, Prog. Polym. Sci., 2010, 35, 3 CrossRef CAS PubMed.
- T. Ueki, S. Sawamura, Y. Nakamura, Y. Kitazawa, H. Kokubo and M. Watanabe, Langmuir, 2013, 29, 13661 CrossRef CAS PubMed.
- P. Techawanitchai, M. Ebara, N. Idota, T. Asoh, A. Kikuchi and T. Aoyagi, Soft Matter, 2012, 8, 2844 RSC.
- J. R. howse, P. Tompham, C. J. Crook, A. J. Gleesom, W. Bras, R. A. L. Hones and A. J. Ryan, Nano Lett., 2006, 6, 73 CrossRef CAS PubMed.
- A. Matsumoto, N. Sato, T. Sakata, R. Yoshida, K. Kataoka and Y. Miyahara, Adv. Mater., 2009, 21, 4372 CrossRef CAS.
- L. Dong and H. Jiang, Soft Matter, 2007, 3, 1223 RSC.
- A. Matsumoto, K. Yamamoto, R. Yoshida, K. Kataoka and Y. Miyahara, Chem. Commun., 2010, 46, 2203 RSC.
- J. Kobayashi and T. Okano, React. Funct. Polym., 2013, 73, 939 CrossRef CAS PubMed.
- R. Yoshida, T. Takahashi, T. Yamaguchi and H. Ichijo, J. Am. Chem. Soc., 1996, 118, 5135 CrossRef.
- R. Yoshida, Adv. Mater., 2010, 22, 3463 CrossRef CAS PubMed.
- R. Yoshida and T. Ueki, NPG Asia Mater., 2014, 6, e107 CrossRef CAS.
- R. Yoshida, T. Sakai, S. Ito and T. Yamaguchi, J. Am. Chem. Soc., 2002, 124, 8095 CrossRef CAS PubMed.
- H. Zhou, Z. Ding, Z. Zhang and Y. Peng, Soft Matter, 2013, 9, 4956 RSC.
- V. V. Yashin, O. Kuksenok and A. C. Balazs, Prog. Polym. Sci., 2010, 35, 155 CrossRef CAS PubMed.
- V. V. Yashin, O. Kuksenok, P. Dayal and A. C. Balazs, Rep. Prog. Phys., 2012, 75, 066601 CrossRef PubMed.
- S. Maeda, Y. Hara, T. Sakai, T. Sakai and R. Yoshida, Adv. Mater., 2007, 19, 3480 CrossRef CAS.
- Y. Shiraki and R. Yoshida, Angew. Chem., Int. Ed., 2012, 51, 6112 CrossRef CAS PubMed.
- (a) T. Ueki, Y. Takasaki, K. Bundo, T. Ueno, Y. Akagi, T. Sakai and R. Yoashida, Soft Matter, 2014, 10, 1349 RSC; (b) T. Ueki and R. Yoshida, Phys. Chem. Chem. Phys., 2014, 16, 10388 RSC.
- Y. Murase, S. Maeda, S. Hashimomto and R. Yoshida, Langmuir, 2009, 25, 483 CrossRef CAS PubMed.
- D. Suzuki, T. Kobayashi, R. Yoahsida and T. Hirai, Soft Matter, 2012, 8, 11447 RSC.
- R. Mitsunaga, K. Okeyoshi and R. Yoashida, Chem. Commun., 2013, 49, 4935 RSC.
- T. Ueki, M. Shibayama and R. Yoshida, Chem. Commun., 2013, 49, 6947 RSC.
- R. Tamate, T. Ueki, M. Shibayama and R. Yoshida, Angew. Chem., Int. Ed., 2014, 53, 11248 CrossRef CAS PubMed.
- T. Masuda, M. Hidaka, Y. Murase, A. M. Akimoto, K. Nagase, T. Okano and R. Yoshida, Angew. Chem., Int. Ed., 2013, 52, 7468 CrossRef CAS PubMed.
- B. M. Peek, G. T. Ross, S. W. Edwards, G. J. Meyer, T. J. Meyer and B. W. Erickson, Int. J. Protein Res., 1991, 38, 114 CrossRef CAS PubMed.
- M. Hidaka and R. Yoshida, J. Controlled Release, 2011, 150, 171 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis measurement, LCST measurement and Arrhenius plot for the BZ reaction. See DOI: 10.1039/c4ra10675j |
| This journal is © The Royal Society of Chemistry 2015 |