Rapid decomposition of Direct Blue 6 in neutral solution by Fe–B amorphous alloys
Yao Tang,
Yang Shao,
Na Chen and
Ke-Fu Yao*
School of Materials Science and Engineering, Tsinghua University, Beijing 100084, People's Republic of China. E-mail: kfyao@tsinghua.edu.cn; Fax: +86-10-62770190; Tel: +86-10-62772292
First published on 5th December 2014
Abstract
The development of environmental-friendly materials for the highly-efficient purification of dye-containing wastewater is important and attractive. In this work, Fe–B amorphous alloy ribbons were fabricated and employed to degrade the azo dye, Direct Blue 6. It shows that Fe–B binary amorphous alloys possess low reaction activation energy (25.43 kJ mol−1) and much higher degrading efficiency than their crystalline counterparts and the commercial iron powders. Under the same experimental conditions (25 °C, initial pH = 7), the surface area normalized reaction rate constant of Fe84B16 amorphous alloy is approximately 1.8 and 89 times as that of its crystalline alloy and the 300 mesh iron powders, respectively. It indicates that the homogeneous amorphous structure is beneficial to the degradation rate of the zero valent iron. It has been found that boron plays an important role in enhancing the degradation rate because it contributes to the formation of an incompact oxide layer at the metal–water interface. Unlike the dense oxide layer formed on the surface of iron powders, this incompact layer would benefit iron atoms' movement and promote the reduction of contaminants. The present results provide a new amorphous zero valent iron material and a new way of designing proper iron-based alloys for waste-water remediation.
Introduction
Azo dyes, containing a series of colourful compounds bearing the functional –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group, are widely used in the textile, paper, leather, food, cosmetic and pharmaceutical industries. Although azo dyes contribute to the colourful world, due to the electron deficiency character of the azo group, these dyes are resistant to mineralization by most aerobic bacteria in the natural world, thus making the treatment of their wastewater difficult. Nowadays, the major techniques to treat dye effluents are biological treatments and activated carbon absorption methods.1 However, there are shortcomings in these techniques, such as the low efficiency of biological treatments, and no decomposition but only transferring the dyes to another place in the activated carbon method.2,3 In the recent years, due to their unique properties and low cost, the reductive degradation of azo dyes by zero-valent iron (ZVI) has attracted considerable attention.
N– group, are widely used in the textile, paper, leather, food, cosmetic and pharmaceutical industries. Although azo dyes contribute to the colourful world, due to the electron deficiency character of the azo group, these dyes are resistant to mineralization by most aerobic bacteria in the natural world, thus making the treatment of their wastewater difficult. Nowadays, the major techniques to treat dye effluents are biological treatments and activated carbon absorption methods.1 However, there are shortcomings in these techniques, such as the low efficiency of biological treatments, and no decomposition but only transferring the dyes to another place in the activated carbon method.2,3 In the recent years, due to their unique properties and low cost, the reductive degradation of azo dyes by zero-valent iron (ZVI) has attracted considerable attention.
ZVI is an environmentally friendly reducing agent and can decompose a series of oxidative hazardous materials.4–7 Nam et al.7 showed that nine azo dyes could be rapidly degraded by granular irons with a first-order rate constant (kobs) of 0.35 ± 0.1 min−1 for Orange II at 130 rpm on an orbital shaker, corresponding to a surface area normalized rate constant (kSA) of 0.21 ± 0.01 L m−2 min−1. However, most of the tests with high degradation efficiency were conducted in anaerobic systems.2,3,8,9 Bigg et al.3 found that if the initial dissolved oxygen reached 9 mg L−1 in the solution pH = 6, the reaction between Acid Blue 113 and iron powders would stop. The main reason for this phenomenon is the significant passivation, and the formed dense oxide layer on the iron powder surface inhibits the electron transfer from the iron matrix to the contaminants. To enhance the degradation efficiency of ZVI in the open air, plating with a second metal to form galvanic cell or adjusting the wastewater into acid conditions are always preferred.10–12 However, the added second metal is either expensive (Pd) or toxic (Ni or Cu) and the acid condition itself is not only another source of pollution but also increases the cost of waste disposal. Therefore, it is important to explore new ways to enhance the degradation efficiency.
It is known that iron-based amorphous alloys is a kind of zero-valent iron material.13,14 In particular, they possess the characteristics of a metastable structure and chemical homogeneity. Thus, iron-based amorphous alloys are more reactive than their crystalline counterparts and chemically reactive on the entire surface rather than the defects such as the grain boundary in crystalline materials. These merits could enable iron-based amorphous alloys to exhibit higher degradation efficiency than the crystalline ZVI (CZVI). In addition, since the discovery in 1970s, iron-based amorphous alloys have been widely studied because of their exceptional soft magnetism and corrosive resistance.15–17 With the development of the fabrication method of melt spinning, iron-based amorphous alloy ribbons became a low cost material and have been commercialized in transformer coils.18,19 Combining these advantageous points, one can expect that the iron-based amorphous alloys (amorphous ZVI, AZVI) may be more capable of decomposing oxidative pollutants compared to CZVI and still have a low cost.
Indeed, the results reported by Zhang et al.20 and Wang et al.21 very recently confirmed that the degradation rate of DB6 with AZVI was obviously higher than their crystalline counterparts and iron powders. It suggests that iron-based amorphous alloys might be a good candidate for replacing CZVI in industrial applications despite the fact that more research is necessary for understanding the related mechanism, the effect of non-iron elements and so on. It is well known that the electron donor in the degradation process is the iron element; however, to obtain a suitable glass forming ability, metalloids and other metals are added into AZVI. Till date, there are no reports about the effect of other elements especially metalloids on AZVI's degradation ability. In order to understand the metalloids' effect on degradation, in this study, the simplest Fe–B binary amorphous alloys have been used to study the decomposition of a DB6 solution and the effects of boron on the superior degradation efficiency are discussed.
Experimental
The Fe–B amorphous ribbons with a thickness of around 30 μm, were fabricated by melt spinning the ingot of Fe–B alloys with the same nominal composition. The detailed synthetic procedure has been described in a previous work.22 Part of the Fe84B16 amorphous ribbons was vacuum-annealed at 773 K for 1 h to obtain their fully crystalline counterpart. To identify their structure, X-ray diffraction (XRD, Rigaku D/max-RB) analysis was performed. A scanning electron microscope (SEM, LEO 1530) was utilized to identify the change of morphology of the ribbons before and after degradation. The Brunauer–Emmett–Teller (BET) surface area analysis of the ribbons and powders was performed using the nitrogen method with a surface analyser model NOVA4000, Quantichrome Instruments, USA. X-ray photoelectron spectroscopy (XPS) analysis was conducted on a Thermo Scientific ESCALAB 250Xi instrument using Al-Kα radiation to identify the composition and chemical status of the amorphous ribbons.Industrial Direct Blue 6 (C32H20N6S4O14Na4, C.I. 22610) with 100% light strength was obtained from Hailan Chemical Pigment Co (Tianjin, China). All other reagents were of analytical reagent grade. Deionized water was used as the solvent throughout this study. In addition, a UV-vis spectrophotometer (UnicoUV-2802PC) was used to identify the azo dye and monitor its concentration change during the decomposition.
The degradation experiments of DB6 were conducted with the amorphous, crystalline ribbons and 300 mesh iron powders, separately. The initial pH of the DB6 solution was measured by a digital pH metric (FE20, Mettler Toledo). All the ribbons and powders were used without pretreatments such as acid washing. In a typical experiment, 240 mL solution (200 mg L−1) and 3.2 g ribbons or powders were added in a 500 mL beaker, which was placed in a temperature controlled water-bath trough and agitated at a fixed speed. At fixed intervals, a 4 mL aliquot was removed with a syringe and filtered with a 0.45 μm membrane. The filtered samples were pipetted out and subjected to UV-vis spectrum scanning.
Results and discussion
To differentiate the amorphous and crystalline iron-based materials utilized in this study, the structure and morphology of the as-prepared Fe84B16 amorphous ribbons, annealed Fe84B16 ribbons and 300 mesh iron powders were examined by XRD and SEM (Fig. 1). As is shown in Fig. 1(a), for the as-prepared ribbon, it is a typical XRD spectrum of amorphous materials – a broad hump without any detectable crystalline peaks. However, for the ribbons annealed at 773 K for 1 h, beside the diffraction peaks of the α-Fe phase, diffraction peaks resulting from the Fe3.5B precipitates are observed. For the commercial 300 mesh iron powders, only diffraction peaks resulting from the α-Fe phase are identified.Fig. 1(b) and (c) are SEM images of the Fe84B16 amorphous ribbons' cross section view and 300 mesh iron powders. It shows that the thickness of the amorphous ribbons is around 30 μm, and their surface (the inset of Fig. 1(b)) is rather smooth, whereas for the 300 mesh iron powders, the average diameter is around 50 μm and the surface is pretty rough. It is well known that a special surface area is essential to the heterogeneous reaction in chemistry. To identify the specific surface area, the BET analysis with nitrogen gas is applied. For the 300 mesh iron powders, the specific surface area was measured to be 0.343 m2 g−1. But, for the ribbons, because their specific surface area is too low and out of the range of the BET analysis with nitrogen, other methods need to be applied to estimate the specific surface area. Considering that the ribbons are pretty smooth, it is reasonable to assume the ribbons as cuboids as was done in a previous study.23 Based on this assumption, the specific surface area of the ribbons is calculated to be 0.0088 m2 g−1. These results indicate the commercial iron powders exhibit much larger specific surface area values than the Fe84B16 ribbons.
The composition as well as the chemical status of the Fe84B16 amorphous alloys was identified using XPS analysis. The results are shown in Fig. 2. The XPS survey scan shows the existence of iron and boron. Through calculations, it was found that after 300 s Ar+ sputtering, the atomic ratio of B to Fe decreased from around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, indicating that on the surface layer the B content is higher than that in the inner layers. Fig. 2(b) shows the Fe 2p spectra, which is split into two lines Fe 2p3/2 and Fe 2p1/2 by about 13.4 eV with a theoretical relative intensity ratio of 2
4, indicating that on the surface layer the B content is higher than that in the inner layers. Fig. 2(b) shows the Fe 2p spectra, which is split into two lines Fe 2p3/2 and Fe 2p1/2 by about 13.4 eV with a theoretical relative intensity ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In this discussion, the Fe 2p3/2 line was investigated. Before sputtering, the binding energies of 706.7, 710.0 and 711.5 eV were assigned to metallic, ferrous and ferric iron, respectively. After sputtering, the binding energies of 706.4 and 707.6 eV were due to the metallic iron and Fe2B. However, for the B 1s spectra shown in Fig. 2(c), the binding energies at 191.4 and 187.7 eV should be oxidized and elemental boron. These results are consistent with previous research, which indicate a different native oxide film than crystalline Fe–B alloys.24
1. In this discussion, the Fe 2p3/2 line was investigated. Before sputtering, the binding energies of 706.7, 710.0 and 711.5 eV were assigned to metallic, ferrous and ferric iron, respectively. After sputtering, the binding energies of 706.4 and 707.6 eV were due to the metallic iron and Fe2B. However, for the B 1s spectra shown in Fig. 2(c), the binding energies at 191.4 and 187.7 eV should be oxidized and elemental boron. These results are consistent with previous research, which indicate a different native oxide film than crystalline Fe–B alloys.24
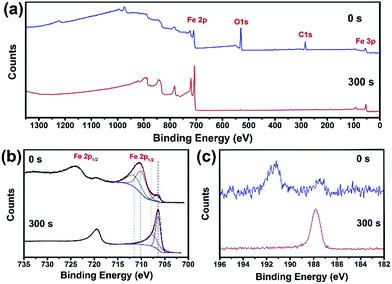 | ||
| Fig. 2 XPS spectra of Fe84B16 amorphous ribbons before and after 300 seconds of Ar+ sputtering (a) XPS survey scan, (b) Fe 2p and (c) B 1s photoelectron spectrum. | ||
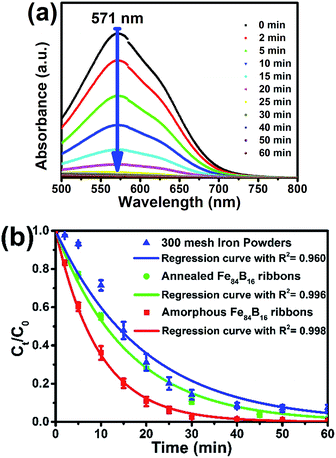
The UV-vis spectra of a DB6 solution recorded after different times are presented in Fig. 3(a), which shows that the Fe84B16 amorphous ribbons could completely decompose the 200 mg L−1 DB6 solution within 30 min. DB6 shows a strong absorbance band at 571 nm due to the n–π* transition of the azo bond, and according to the absorption intensity at 571 nm, the concentration variation against time was obtained and is shown in Fig. 3(b). It shows that the concentration of DB6 decreases rapidly with all three kinds of iron materials. Through regression, it has been found that the degradation of DB6 with all the three kinds of materials follows the first order kinetic model as shown in eqn (1), similar to other reported results:2,7,23
| Ct/C0 = exp(−kobs/t) | (1) |
As is shown in Table 1, the Fe84B16 amorphous ribbons exhibit 2 times and 10 times higher degradation rate than the Fe84B16 annealed ribbons and 300 mesh commercial iron powders.
| Materials | Area dosagea (m2 L−1) | kobs (min−1) | Half-lifeb (min) | kSA (L m−2 min−1) |
|---|---|---|---|---|
a All the areas are calculated by assuming the shape as a sphere or cuboid and the density data of all the amorphous ribbons cited from ref. 27.b Half-life is calculated by kobs with the equation t1/2 = (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/kobs. 2)/kobs. |
||||
| 300 mesh iron powders | 4.573 | 0.049 | 14.1 | 0.011 |
| Crystalline Fe84B16 ribbons | 0.112 | 0.062 | 11.2 | 0.554 |
| Amorphous Fe84B16 ribbons | 0.112 | 0.110 | 6.30 | 0.982 |
| Amorphous Fe82B18 ribbons | 0.125 | 0.113 | 6.13 | 0.904 |
| Amorphous Fe80B20 ribbons | 0.160 | 0.156 | 4.44 | 0.975 |
For surface processes the reaction rate (r) could be described by the Langmuir–Hinshelwood equation where rate is proportional to the surface coverage (θ).25
| r = kθ = kKC(1 + kC) | (2) |
With a fast reaction at the surface and low solution concentration, only a small number of reactive sites is occupied (kC ≪ 1) and the rate could be described by a pseudo-first-order reaction: r = kKC = k′C and the integral equation is shown in eqn (1).
From this supposition, reactive sites should not be the limited reason for the degradation, and to explore the interior degradation ability of different iron materials; according to Nam et al.,7 the observed reduction rate constant of an azo dye should be divided by the iron surface area concentration (ρa = S/V, S is the total surface of ZVI and V is the volume of the solution), namely, kobs = kSAρa, and kSA is the surface area normalized first order rate constant.7 The calculated kSA values (Table 1) of the present amorphous and crystalline ribbons is approximately 89 and 50 times that of the 300 mesh iron powders. It indicates that both the metastable amorphous structure and the boron addition would have played an important role in enhancing the reductive activity of ZVI.
To fulfil the kinetic analysis and identify the activation energy, batch experiments were carried out in the range of 25–55 °C for the Fe84B16 amorphous ribbons. As is shown in Fig. 4, with increasing experimental temperature, the degradation rate of DB6 by Fe84B16 amorphous ribbons increases significantly. After regression with eqn (1), the first-order rate constant of the degradation process at different temperatures, kT, was obtained. Thus, the activation energy can be derived from the Arrhenius-type eqn (3):8,23
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kT = −ΔE/RT + ln kT = −ΔE/RT + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A A
| (3) |
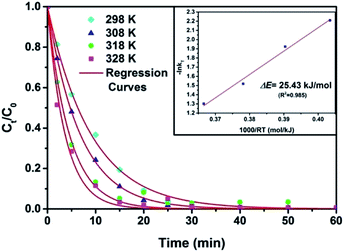 | ||
Fig. 4 Temperature effect on the decomposition of DB6 with Fe84B16 amorphous ribbons. Inset: Arrhenius plot of −ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k versus 1000/RT for the decomposition. k versus 1000/RT for the decomposition. | ||
The similarity in kinetics suggests that the degradation pathway of the DB6 solution with amorphous ribbons could be the same as that of the crystalline iron scraps. In order to confirm this speculation, the morphology and structure of the ribbons and the sediments after degradation were examined by SEM and XRD (Fig. 5). Although the smooth surface becomes rough after degradation, the structure of the ribbons remains amorphous (the inset of Fig. 5(a)). According to Fig. 5(b), the sediments after degradation were confirmed to be γ-FeOOH and NaSO4, which are consistent with the products of the reaction between the crystalline iron plates and Orange II.25 Taking into account that the incident depth of the XRD is only around 0.5–5 μm and no diffraction peak of γ-FeOOH could be found in the inset of Fig. 4(a), all the reactions are supposed to occur on the surface of the amorphous ribbons and the formed products are exfoliated from the matrix during the agitation.
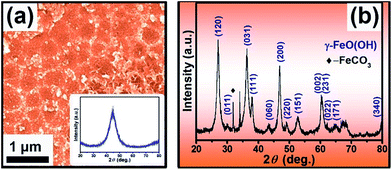 | ||
| Fig. 5 (a) SEM image and XRD spectrum (inset) of amorphous ribbons after degradation; (b) XRD spectrum of the sediments. | ||
In combination with the UV-vis spectra of DB6 as is shown in Fig. 1(a), the degradation pathway of DB6 with Fe–B amorphous alloys should be the same as the one with crystalline ZVI widely studied before, namely, the reductive cleavage of the azo bond (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–).
N–).
Moreover, according to the low activation energy of the Fe–B amorphous alloys, it could be deduced that the determining factor is the diffusion step of the contaminants to the iron matrix. As illustrated by Scherer,28 according to their different structures, the layer of oxides could act as a physical barrier, semiconductor or coordinating surface. A thick and compact oxide layer, which is always found on the surface of CZVI, could act as a physical barrier to inhibit the electron transfer and lead to the decrease of reactivity.29 Then, the different oxide film structures could be the key factor to affect the degradation rate of DB6 with different ZVI materials.
Based on the study of the passive film in corrosion science, the oxide film or passive film is affected by the structure and the composition of the matrix.13,14 According to the research on the structure's effect on the native oxide film done by Sharma,24 the thickness of the native oxide film on the amorphous Fe75B25 ribbons is close to that of the single phase Fe3B but only one third of that of the annealed binary phase ribbons consists of α-Fe and Fe2B, which means the homogeneous amorphous structure inhibits the formation of a thick oxide film. Based on the Ponder's electrochemistry experiment on Fe–B alloys, due to the even lower standard potential of boron (E0 = −0.87 V vs. SHE), boron plays a central role in the corrosive behaviour and the formed HBO2 or H3BO3 or an amorphous mixture with the iron oxide on the surface also affects the initial passive corrosion activity.30 Considering the solubility of borate, it is reasonable to believe that the addition of boron will inhibit the formation of a dense oxide layer at the metal–water interface. The formed incompact oxide layer would decrease the obstacle of the electron transfer from the metal to the pollutant and results in the high kSA value as well as low activation energy of the Fe–B amorphous alloys. Thus, although the specific surface area of the commercial iron powders are much larger than that of the Fe84B16 ribbons, both the amorphous and crystalline ribbons exhibit a higher kobs and lower half-life than the 300 mesh iron powders.
In addition, as is shown in Table 1, if the size of the Fe–B amorphous or crystalline ribbons could be reduced significantly, such as in the nanometer scale, they would exhibit a much higher observed degradation rate than the current results. For example, if the Fe84B16 ribbons, either amorphous or crystalline, could be fabricated into spherical particles with diameters of around 1 μm, the calculated surface area of the 3.2 g powders would be 1.917 m2, and the corresponding area dosage (ρa) as in present study would be 7.985 m2 L−1. When the ρa was multiplied with the kSA, the kobs of the amorphous and crystalline Fe84B16 ribbons would be as large as 7.841 and 4.423 min−1, respectively, which means that the half-life of DB6 would be as short as 5.3 and 9.4 s. It indicates that decreasing the size of the Fe–B amorphous or crystalline materials would significantly shorten the degradation time and boron addition should be a promising way to enhance the degradation rate of ZVI.
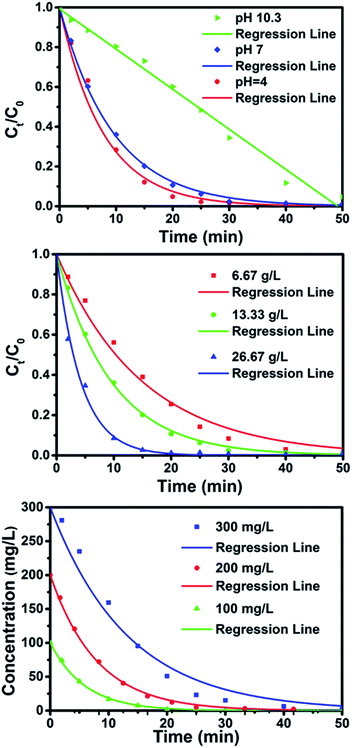
It is widely known that environmental factors, such as pH, contaminant concentration and iron usage, have great impact on the decomposition process of ZVI. However, to date, there is no detailed report about the effects of these factors on the decomposition of contaminants with amorphous iron materials. Thus, in this study, beside the temperature as is shown in Fig. 3, environmental variables, including solution pH, dosage of Fe84B16 amorphous ribbons and DB6 concentration on the degradation were utilized to investigate their effects on the reaction rate and decomposition efficiency of DB6 by Fe–B amorphous ribbons, as is shown in Fig. 6.
Solution pH has been experimentally proven to be an important factor in iron-contaminant systems.8 For most dye wastewater, their pH is in the range of 6–10. In this work, therefore, the effect of initial solution pH on the decomposition of DB6 by Fe84B16 has been studied in a pH range of 4–10, and the results are shown in Fig. 6(a). With the initial pH at 4, 7, the DB6 solution was completely decomposed within 30 minutes. As the initial pH increased up to 10, the reaction rate fits with the pseudo-zero-order reaction. This could stem from the fact that at lower pH (<pHpzc ≈ 8, pzc stands for the point of zero charge), the surface of iron is positively charged, which is favorable for the adsorption of the dye molecules onto the iron surface because dye molecules with a sulfuric group are negatively charged. When the solution pH is above the isoelectric point, the oxide surface becomes negatively charged and the surface could be easily covered by corrosion products. However, even at the initial pH 10, more than 90% degradation efficiency can be obtained within 50 minutes. This result has a very practical meaning. It implies that there is no need to add any acid into the reaction system continuously to keep the acid condition.
Fig. 6(b) shows that the Fe84B16 dosage has significant influence on the degradation rate. With increasing the Fe84B16 dosage from 6.67 to 26.67 g L−1, the time required for complete decomposition of DB6 was reduced from over 50 minutes to about 20 minutes. Through regression, the observed reaction rate constants, kobs, were obtained, which increased from 0.067 to 0.237 min−1. It shows that the observed reaction rate is increased by about 2.5 times when the Fe84B16 dosage used is increased 3 times. Thus, it is practical to employ a slightly higher dosage of iron-based amorphous alloys, which would shorten the treatment time.
Fig. 6(c) shows the variation of the concentration versus degradation time in the solution with the initial DB6 concentration of 100 mg L−1, 200 mg L−1 and 300 mg L−1. Unlike the effect of lowering degradation efficiency observed in high concentration Orange II by nano-scale iron powders,8 all the contaminants were almost fully decomposed by the amorphous Fe84B16 ribbons within about 50 minutes, despite the time used was increased from about 20 to about 50 minutes. All of the above results indicate that the Fe–B amorphous ribbon is a good candidate for field applications in the degradation of azo dye containing wastewater.
Conclusions
Fe84B16 amorphous alloy ribbons with a thickness of around 30 μm were fabricated to degrade 200 mg L−1 DB6 neural solutions under mild conditions in open air. It has been found that the Fe84B16 amorphous ribbons completely decomposed the 200 mg L−1 DB6 solution within only 30 min. Under the same conditions, the amorphous ribbons exhibit a lower reaction activation energy (25.43 kJ mol−1) and higher observed degradation rate constant (0.110 min−1) at 25 °C than the crystalline ribbons and 300 mesh commercial iron particles. The surface area normalized reaction rate constant of the Fe84B16 amorphous ribbons is approximately 1.8 and 89 times that of its crystalline ribbons and the 300 mesh commercial iron powders, indicating that the degradation efficiency of AZVI in decomposing azo dyes is much higher than that of CZVI. The degradation mechanism of the amorphous Fe84B16 is reductive degradation, which is the same as that of CZVI. It shows that the high degradation efficiency of the Fe–B amorphous ribbons results from its amorphous structure and the boron addition, which inhibits the formation of a thick and compact oxide layer on the surface. The experiments with different environmental parameters further proved the high efficiency of Fe–B amorphous alloys in the degradation of azo dyes. The present study indicates that Fe–B amorphous alloy ribbons possess high reductive activity and can serve as a new kind of ZVI material to decompose oxidative contaminants.Acknowledgements
This work was supported by the National Science Foundation of China (Grand no. 51271095).Notes and references
- J. R. Perey, P. C. Chiu, C. P. Huang and D. K. Cha, Water Environ. Res., 2002, 74(3), 221–225 CrossRef CAS.
- J. S. Cao, L. P. Wei, Q. G. Huang, L. S. Wang and S. K. Han, Chemosphere, 1999, 38(3), 565–571 CrossRef CAS.
- T. Bigg and S. J. Judd, Process Saf. Environ. Prot., 2001, 79, 297–303 CrossRef CAS.
- R. W. Gillham and S. F. Ohannesin, Ground Water, 1994, 32(6), 958–967 CrossRef CAS PubMed.
- L. J. Matheson and P. G. Tratnyek, Environ. Sci. Technol., 1994, 28(12), 2045–2053 CrossRef CAS PubMed.
- H. Ma, Y. P. Huang, M. W. Shen, D. M. Hu, H. Yang and M. F. Zhu, et al., RSC Adv., 2013, 3, 6455–6465 RSC.
- S. Nam and P. G. Tratnyek, Water Res., 2000, 4(6), 1837–1845 CrossRef.
- J. Fan, Y. H. Guo, J. J. Wang and M. H. Fan, J. Hazard. Mater., 2009, 166(2–3), 904–910 CrossRef CAS PubMed.
- M. F. Hou, F. B. Li, X. M. Liu, X. G. Wang and H. F. Wan, J. Hazard. Mater., 2007, 145, 305–314 CrossRef CAS PubMed.
- T. Satapanajaru, C. Chompuchan, P. Suntornchot and P. Pengthamkeerati, Desalination, 2011, 266(1–3), 218–230 CrossRef CAS PubMed.
- S. H. Chang, K. S. Wang, S. J. Chao, T. H. Peng and L. C. Huang, J. Hazard. Mater., 2009, 166, 1127–1133 CrossRef CAS PubMed.
- P. X. Wu, C. M. Liu, Z. J. Huang and W. M. Zhang, RSC Adv., 2014, 4, 25580–25587 RSC.
- R. Hasegawa, in Glassy metals: magnetic, chemical and structural properties, CRC Press, Boca Raton, 1983 Search PubMed.
- F. E. Luborsky, Amorphous metallic alloys, Butterworth and Co Ltd, 1983 Search PubMed.
- C. Suryanarayana and A. Inoue, Int. Mater. Rev., 2013, 58(3), 131–166 CrossRef CAS PubMed.
- K. F. Yao and C. Q. Zhang, Appl. Phys. Lett., 2007, 90, 061901 CrossRef PubMed.
- F. J. Liu, K. F. Yao and H. Y. Ding, Intermetallics, 2011, 19, 1674–1677 CrossRef CAS PubMed.
- R. Hasegawa and R. Ray, J. Appl. Phys., 1978, 49(7), 4174–4179 CrossRef CAS PubMed.
- Y. Yoshizawa, S. Oguma and K. Yamauchi, J. Appl. Phys., 1988, 64(10), 6044–6046 CrossRef CAS PubMed.
- C. Q. Zhang, H. F. Zhang, M. Q. Lv and Z. Q. Hu, J. Non-Cryst. Solids, 2010, 356(33–34), 1703–1706 CrossRef CAS PubMed.
- J. Q. Wang, Y. H. Liu, M. W. Chen, G. Q. Xie, D. V. Louzguine-Luzgin and A. Inoue, et al., Adv. Funct. Mater., 2012, 22(12), 2567–2570 CrossRef CAS.
- H. Y. Ding, W. Zhang and K. F. Yao, Mater. Sci. Forum, 2013, 745–746, 722–727 CrossRef.
- C. Q. Zhang, Z. W. Zhu, H. F. Zhang and Z. Q. Hu, J. Non-Cryst. Solids, 2012, 358, 61–64 CrossRef CAS PubMed.
- S. K. Sharma and S. Hofman, Appl. Surf. Sci., 1991, 51, 139–155 CrossRef CAS.
- J. A. Mielczarski, G. M. Atenas and E. Mielczarski, Appl. Catal., B, 2005, 56(4), 289–303 CrossRef CAS PubMed.
- J. Chen and L. Zhu, Catal. Today, 2007, 126(3–4), 463–470 CrossRef CAS PubMed.
- R. Ray, R. Hasegawa, C. P. Chou and L. A. Davis, Scr. Metall., 1977, 11, 973–978 CrossRef CAS.
- D. L. Sparks and T. J. Grundl, Mineral-water interfacial reactions: kinetics and mechanisms, American Chemical Society, 1999, pp. 301–322 Search PubMed.
- C. Noubactep, J. Hazard. Mater., 2007, 148(3), 773–774 CrossRef CAS PubMed.
- S. M. Ponder, J. G. Darab, J. Bucher, D. Caulder, I. Craig and L. Davis, et al., Chem. Mater., 2001, 13, 479–486 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |