Synthesis and catalytic activity of carbon supported copper nanoparticles for the synthesis of aryl nitriles and 1,2,3-triazoles
Abstract
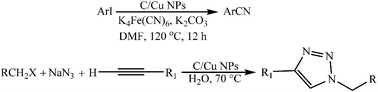
Various substituted aryl nitrile and 1,2,3-triazole derivatives were prepared by using carbon supported copper nanoparticles (C/Cu NPs) as a heterogeneous catalyst under ligand-free conditions, which provided good to excellent yields. The nanocatalyst can be recycled and reused several times without significant loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...