Highly organized nanofiber formation from zero valent iron nanoparticles after cadmium water remediation
Abstract
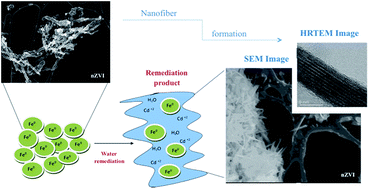
Many studies have used nanoscale zero valent iron (nZVI) nanoparticles to remove redox-sensitive metals (e.g., As, Cr, U, Se, Ni, Cu) from aqueous systems by absorption or reduction processes. However, very few investigations present a detailed study of the product formed after the remediation process. In order to quantify the efficiency of nZVI particles as a possible cadmium remediation agent, we prepared nZVI by sodium borohydride reduction of an iron complex, FeCl3·6H2O, at room temperature and ambient pressure. Fe0 and nanocrystalline structures of iron oxides and oxyhydroxides were obtained with this method. We exposed the nZVI to 6 ppm of Cd2+ and characterized the products with X-ray diffraction, X-ray absorption and X-ray photoelectron spectroscopy. Inductively coupled plasma analysis showed that the nZVI remediation efficiency of cadmium ions was between 80% and 90% in aqueous media. All of the physical characterization results confirmed the presence of Fe0, α-Fe2O3 and FeOOH. High resolution transmission electron microscopy images showed nanofiber formation of a mixture of Fe0, oxyhydroxides and oxides iron formed after interacting with cadmium ions, possibly forming CdFe2O4. These results suggest that the FeOOH shell and other iron oxides in nZVI could enhance Cd2+ removal. This removal is observed to cause a change of the initial structure of nZVI to nanofibers due to possible formation of CdFe2O4 as a waste product.

 Please wait while we load your content...
Please wait while we load your content...