Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two-photon uncageable enzyme inhibitors bearing targeting vectors†
Philipp
Anstaett
a,
Vanessa
Pierroz
ab,
Stefano
Ferrari
b and
Gilles
Gasser
*a
aDepartment of Chemistry, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland. E-mail: gilles.gasser@chem.uzh.ch; Fax: +41 44 635 6802
bInstitute of Molecular Cancer Research, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland
First published on 20th August 2015
Abstract
The activity of two cyclooxygenase-2 enzyme inhibitors, Celecoxib and Lumiracoxib, could be suppressed by coupling to photo-labile protecting groups, so-called photocages. These groups could be further functionalized with a peptide targeting vector for specific cellular delivery. The enzyme inhibition potential of the cyclooxygenase-2 inhibitors could be regained upon two-photon excitation with tissue-transparent near-IR light at 800 nm.
Enzymes are important drug targets, and thus understanding the (indirect) outcome of their inhibition is of fundamental significance.1,2 It would therefore be highly desirable to have biochemical tools, which allow for spatio-temporally controlled release of enzyme inhibitors in living cells or organisms. Photo-labile protecting groups (PLPGs), also known as photocages, have been shown in different fields of research to enable the release of molecules of interest upon UV-irradiation.3–8 Very importantly, two-photon (TP) cages have recently allowed for the release of compounds upon simultaneous TP excitation in the near-IR range.9 Consequently, higher spatial precision (sub-cellular) and deeper tissue penetration are possible.10 A few caged enzyme inhibitors with the potential to be two-photon-uncaged have been reported.11–14 However, for those compounds, either no biological TP experiments were performed,13 the irradiation times needed were very long (several hours),11,12 the enzyme activity upon uncaging was only moderately changed,11 or the uncageable concentrations were physiologically irrelevant.14 In this article, we fill this gap and report the TP controlled inhibition of cyclooxygenase-2 (COX-2) by the inhibitors Celecoxib and Lumiracoxib.
Based on a TP cage developed by Goeldner and co-workers,15 which has already been used on HeLa cells,16 we developed PLPGs 1 and 2 (see Scheme 1, and ESI† for their synthesis) to cage Celecoxib and Lumiracoxib. Of note, although the main effect of the two compounds is COX-2 inhibition, their indirect effects are quite different. Lumiracoxib is active against certain cancer cells, while Celecoxib is not. The reasons behind this difference are not yet understood. This highlights even further the need for novel biochemical tools as the one developed in this study. Importantly, in view of potential selective drug delivery, we designed the cage structures to allow for the sub-sequent attachment of targeting vectors, as previously performed by our group for a single photon caged Re complex.17 The carboxylic acid of Lumiracoxib and sulfonamide function of Celecoxib were caged to the alcohol and carboxylic acid moieties of 1 and 2, respectively, to give caged Lumiracoxib 3 and caged Celecoxib 4, respectively. Other cage structures with carboxylic acid attachment groups have been reported before,18 but to the best of our knowledge, 2 is the first biphenyl nitro cage capable of caging via a carboxylic acid function. Consequently, a modified reaction mechanism has to be assumed, possibly in analogy to the uncaging mechanism postulated for 7-nitroindolinyl based groups, with subsequent decarboxylation.19 The biphenyl-core of both cages carries a tetraethylene glycol chain to increase solubility in aqueous media, a critical parameter for the hydrophobic TP cages.20 The terminal azide group can be used to additionally attach any alkyne-containing targeting biomolecules via click chemistry. This gives the opportunity to target the biological effect with two orthogonal methods, i.e. (1) by directing the caged compound towards the intended tissue or organelle with the targeting vector and (2) by light activation. Importantly, the attachment of the targeting moiety to the caged compounds is by design the last step in the synthesis and hence conveniently adjustable. To exemplify this, a peptide, which was shown to bind to annexin1 and to function as a targeting moiety towards certain cancer cells in mice,21 was attached to caged Celecoxib 4, producing targeted caged Celecoxib 5 (see Scheme 1).
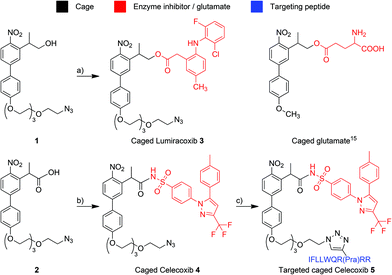 | ||
Scheme 1 Caging of the COX-2 selective inhibitors Lumiracoxib (top) and Celecoxib (bottom), exemplary attachment of a targeting peptide to caged Celecoxib (bottom right), and the previously reported caged glutamate (top right).15 Reaction conditions: (a) N,N′-dicyclohexylcarbodiimide, DMAP, Lumiracoxib, CH2Cl2, 0 °C to rt, 20 h, 80%; (b) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, DMAP, Celecoxib, CH2Cl2, rt, 2 h, 54%; (c) CuSO4, sodium ascorbate, IFLLWQR(Pra)RR, THF/H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, rt to 60 °C, 30 h. DMAP: 4-dimethylaminopyri-dine; Pra: (S)-2-amino-4-pentynoic acid. 1, rt to 60 °C, 30 h. DMAP: 4-dimethylaminopyri-dine; Pra: (S)-2-amino-4-pentynoic acid. | ||
The hydrolytic stability of 3, 4 and 5 in the dark at room temperature was monitored by HPLC and UV/Vis. After 24 h in solution (acetonitrile/PBS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.4), no degradation was observed. The photo-physical properties were characterized and the results are summarized in Table 1. Notably, although photo-degradation of azides to reactive nitrene groups was previously reported,22 such reactions were never observed for 3 or 4, neither upon UV nor fs-pulsed near-IR irradiation. The single photon uncaging quantum yields were determined upon irradiation with a frequency-tripled Nd-YAG laser at 355 nm with azobenzene as reference, as discussed in recent articles on the accurate determination of uncaging quantum yields.23–25 The caged glutamate by Goeldner et al. (Scheme 1) was shown to have a quantum yield of 0.1 at 313 nm.15 For 3, which links the bio-active compound also via an ester to the same cage chromophore, a similar uncaging quantum yield of 0.094 was determined. 4, which has a sulfonamide as linking group, has a significantly lower quantum yield of 0.013. The quantum yield of 5 is with 0.0047 in the range of 4, but still smaller. This difference demonstrates a moderate effect of the targeting moiety on the single-photon uncaging. Low single-photon uncaging rates are in principle desired for two-photon cages since unwanted photolysis under ambient conditions is suppressed. However, in this case, the differences between the compounds are mostly due to the chemical reactions following photo-excitation since the biphenylnitro core is the same for all three compounds. Consequently, also lower TP uncaging action cross-sections (δaΦu) can be expected for 4 and 5, compared to 3. δaΦu were determined upon irradiation at 800 nm with a fs-pulsed laser with a 5 kHz repetition rate using the known reference 7-hydroxycoumarin-4-ylmethyl acetate.26,27 As expected, δaΦu of 4 (0.063 GM) and 5 (0.053 GM) were found to be lower than the one of 3 (0.37 GM). Importantly, the attachment of the targeting peptide was found to not majorly influence the TP uncaging efficiency. As for the single photon uncaging, the value for 3 is in the same range as the one for caged glutamate.15 Notably, δaΦu of a dye attached to the same biphenylnitro cage core was shown to be more than an order of magnitude greater at wavelengths around 740 nm.16 Thus, using lower wavelength light would likely also lead to a more efficient release. However, with respect to future applications, the better tissue transparency at 800 nm28 suggests that investigations at this wavelength are more significant.
1, pH = 7.4), no degradation was observed. The photo-physical properties were characterized and the results are summarized in Table 1. Notably, although photo-degradation of azides to reactive nitrene groups was previously reported,22 such reactions were never observed for 3 or 4, neither upon UV nor fs-pulsed near-IR irradiation. The single photon uncaging quantum yields were determined upon irradiation with a frequency-tripled Nd-YAG laser at 355 nm with azobenzene as reference, as discussed in recent articles on the accurate determination of uncaging quantum yields.23–25 The caged glutamate by Goeldner et al. (Scheme 1) was shown to have a quantum yield of 0.1 at 313 nm.15 For 3, which links the bio-active compound also via an ester to the same cage chromophore, a similar uncaging quantum yield of 0.094 was determined. 4, which has a sulfonamide as linking group, has a significantly lower quantum yield of 0.013. The quantum yield of 5 is with 0.0047 in the range of 4, but still smaller. This difference demonstrates a moderate effect of the targeting moiety on the single-photon uncaging. Low single-photon uncaging rates are in principle desired for two-photon cages since unwanted photolysis under ambient conditions is suppressed. However, in this case, the differences between the compounds are mostly due to the chemical reactions following photo-excitation since the biphenylnitro core is the same for all three compounds. Consequently, also lower TP uncaging action cross-sections (δaΦu) can be expected for 4 and 5, compared to 3. δaΦu were determined upon irradiation at 800 nm with a fs-pulsed laser with a 5 kHz repetition rate using the known reference 7-hydroxycoumarin-4-ylmethyl acetate.26,27 As expected, δaΦu of 4 (0.063 GM) and 5 (0.053 GM) were found to be lower than the one of 3 (0.37 GM). Importantly, the attachment of the targeting peptide was found to not majorly influence the TP uncaging efficiency. As for the single photon uncaging, the value for 3 is in the same range as the one for caged glutamate.15 Notably, δaΦu of a dye attached to the same biphenylnitro cage core was shown to be more than an order of magnitude greater at wavelengths around 740 nm.16 Thus, using lower wavelength light would likely also lead to a more efficient release. However, with respect to future applications, the better tissue transparency at 800 nm28 suggests that investigations at this wavelength are more significant.
| Compound | Φ (λ = 355 nm)a | δ a Φ u (λ = 800 nm)b |
|---|---|---|
| a Acquired with a Nd-YAG laser, relative to the photoisomerizaion of azobenzene.23–25 b Acquired with a fs-pulsed laser with 5 kHz repetition rate relative to 7-hydroxycoumarin-4-ylmethyl acetate;26,27 for further details on both methods, see ESI. | ||
| Caged glutamate | 0.1 (λ = 313 nm)15 | 0.45 GM |
| 3 | 0.094 ± 0.02 | 0.37 ± 0.04 GM |
| 4 | 0.013 ± 0.001 | 0.063 ± 0.008 GM |
| 5 | 0.0047 ± 0.0004 | 0.053 ± 0.008 GM |
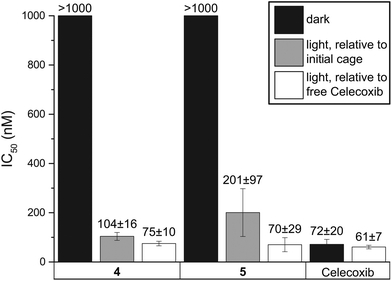
In a study which investigated TP uncaging at 740 nm, it was stated that δaΦu of at least 0.1 GM are required for biological applications.26 Accordingly, the TP uncaging of 4 and 5 could be insufficient for biological studies. However, for aforementioned reasons, it cannot be taken for granted that this is valid at 800 nm. The minimum value certainly depends on the wavelength. Furthermore, additional laser properties, such as the repetition rate of the laser, play a role. Most TP uncaging studies used lasers with MHz repetition rates.27,29 However, 103 smaller repetition rates (kHz), like in this study, have also been used before.15 With the same average powers and peak lengths, the peak photon densities differ therefore by the same factor of 103. Thus, the commonly quoted paradigm that TP uncaging only occurs in the focal point of a laser beam is not generally true. Indeed, we, and others before,15 found that with a laser beam like the one used in this study it is not necessary to focus the beam since the photon density is sufficient with a collimated beam. Furthermore, undesired light phenomena such as white light generation, which was observed if the laser beam was focused, are avoided with a collimated laser beam. These remarks raise the question if biologically relevant uncaging is still possible with TP uncaging cross-sections below 0.1 GM at 800 nm, and if biological structures can withstand such powerful irradiation. To this end, the inhibition potential of COX-2 by the caged compounds 4 and 5, whose uncaging efficiencies are clearly below 0.1 GM, was tested with a fluorescent inhibitor screening assay (see Fig. 1). An irradiation time of 15 min was chosen since this time frame was used in biological studies before.20,30 Notably, this time is significantly shorter than what was required in other reports on uncaging of drugs.11,12,31 To test if the high photon densities influence biomolecules, the enzyme was irradiated using these experimental conditions and its activity tested in comparison to a non-irradiated sample. The activity of the enzyme was found to be unaffected by the pulsed laser light. In addition, Celecoxib was irradiated. No decomposition could be detected by HPLC analysis and its inhibitory potential towards COX-2 remained unchanged at around 60–70 nM. Likewise, solutions of the caged compounds 4 and 5 were tested. Before uncaging, no inhibition of COX-2 could be detected within the limits given by the solubility of the caged compounds. After 15 min of irradiation, the amount of free Celecoxib was determined by HPLC. The uncaging progress was around 70% for 4 and 40% for 5. The IC50 values relative to the released Celecoxib are for both 4 and 5 identical to the non-caged Celecoxib. Relative to the caged starting materials, the IC50 values are slightly higher due to the incomplete release in the given timeframe. Nevertheless, at least five- and ten-fold increases for 4 and 5, respectively, were observed.
To assess if the targeting vector afforded the expected selectivity towards specific cancer cells, we prepared a derivative of 5, which contains an additional fluorescein moiety (16, see ESI† for structure and experimental details). By monitoring fluorescence, we could follow the uptake of the bioconjugate into cells. To this end, we selected A549 human lung adenocarcinoma epithelial cells, which express high levels of annexin1, and HEK 293 human embryonic kidney cells, which have low levels of annexin1 expression.32,33 Unfortunately, the anticipated preferential uptake of 16 into A549 cells could not be observed (see Fig. S8 and S9†). In a previous study of our group, a luminescent rhenium complex was shown to be taken better up by cells targeted with a peptide attached to a related single photon cage. This suggests that, possibly, biasing caused by the luminescent label itself could be responsible for this observation.34 An effect based on the two-photon cage itself can, however, not be ruled out completely. Nevertheless, this does not alter the fact that the photocages presented in this article allow for biomolecules to be easily attached.
In summary, we could demonstrate that targetable TP uncaging can be employed to control enzyme activity. The two drugs Celecoxib and Lumiracoxib, which both selectively inhibit COX-2, were deactivated by conjugation to TP cages. A targeting peptide was attached to the cage. Importantly, the peptide did neither significantly alter photorelease nor enzyme inhibition. The uncaging process was shown to allow for efficient control over the inhibition of an enzyme with near-IR light in an in vitro assay. The concept holds great promise for the future, not only for chemical biological studies on enzyme function, but potentially also for applications in therapeutic targeted drug delivery. Currently, Photodynamic Therapy (PDT) is the most prominent method which utilizes light in a medicinal context in the treatment of certain skin conditions and cancers.35–37 PDT, however, depends on the generation of reactive oxygen species, although many cancerous tissues are hypoxic.38,39 Therapeutic TP uncaging, with the ability to activate virtually any drug without depending on additional factors such as oxygen, could therefore further promote non-invasive light based therapies.
This work was supported by the Swiss National Science Foundation (Professorships No PP00P2_133568 and PP00P2_157545 to G. G.), the University of Zurich (G. G.), the Stiftung für Wissenschaftliche Forschung of the University of Zurich (G. G. and S. F.), the Stiftung für Krebsforschung (S. F.), the Huggenberger-Bischoff Stiftung (S. F.), and the Hartmann Müller-Stiftung (S. F.). The authors would like to thank Prof. Peter Hamm, Dr Julien Réhault and especially Klemens Koziol for help and support with the two-photon uncaging experiments, Dr Anita G. Schmitz for helpful discussions and the Center for Microscopy and Image Analysis of the University of Zurich for access to state-of-the-art equipment.
Notes and references
- H. Nam, N. E. Lewis, J. A. Lerman, D.-H. Lee, R. L. Chang, D. Kim and B. O. Palsson, Network Context and Selection in the Evolution to Enzyme Specificity, Science, 2012, 337, 1101–1104 CrossRef CAS PubMed.
- P. K. Mazur, N. Reynoird, P. Khatri, P. W. T. C. Jansen, A. W. Wilkinson, S. Liu, O. Barbash, G. S. Van Aller, M. Huddleston, D. Dhanak, P. J. Tummino, R. G. Kruger, B. A. Garcia, A. J. Butte, M. Vermeulen, J. Sage and O. Gozani, SMYD3 links lysine methylation of MAP3K2 to Ras-driven cancer, Nature, 2014, 510, 283–287 CrossRef CAS PubMed.
- C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Light-Controlled Tools, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef CAS PubMed.
- H.-M. Lee, D. R. Larson and D. S. Lawrence, Illuminating the Chemistry of Life: Design, Synthesis, and Applications of “Caged” and Related Photoresponsive Compounds, ACS Chem. Biol., 2009, 4, 409–427 CrossRef CAS PubMed.
- G. C. R. Ellis-Davies, Caged compounds: photorelease technology for control of cellular chemistry and physiology, Nat. Methods, 2007, 4, 619–628 CrossRef CAS PubMed.
- D. K. Sinha, P. Neveu, N. Gagey, I. Aujard, C. Benbrahim-Bouzidi, T. Le Saux, C. Rampon, C. Gauron, B. Goetz, S. Dubruille, M. Baaden, M. Volovitch, D. Bensimon, S. Vriz and L. Jullien, Photocontrol of Protein Activity in Cultured Cells and Zebrafish with One- and Two-Photon Illumination, ChemBioChem, 2010, 11, 653–663 CrossRef CAS PubMed.
- A. A. Kumbhar, A. T. Franks, R. J. Butcher and K. J. Franz, Light uncages a copper complex to induce nonapoptotic cell death, Chem. Commun., 2013, 49, 2460–2462 RSC.
- C. Gwizdala and S. C. Burdette, in Inorganic Chemical Biology: Principles, Techniques and Applications, ed. G. Gasser, John Wiley & Sons, Ltd, Chichester, 2014, ch. 9, pp. 275–308 Search PubMed.
- T. M. Dore, in Dynamic studies in biology. Phototriggers, photoswitches and caged biomolecules, ed. M. Goeldner and R. Givens, Wiley-VCH, Weinheim, 2005, ch. 8, pp. 435–459 Search PubMed.
- E. B. Brown, J. B. Shear, S. R. Adams, R. Y. Tsien and W. W. Webb, Photolysis of Caged Calcium in Femtoliter Volumes Using Two-Photon Excitation, Biophys. J., 1999, 76, 489–499 CrossRef CAS.
- H. J. Montgomery, B. Perdicakis, D. Fishlock, G. A. Lajoie, E. Jervis and J. Guy Guillemette, Photo-Control of nitric oxide synthase activity using a caged isoform specific inhibitor, Bioorg. Med. Chem., 2002, 10, 1919–1927 CrossRef CAS.
- B. Perdicakis, H. J. Montgomery, G. L. Abbott, D. Fishlock, G. A. Lajoie, J. G. Guillemette and E. Jervis, Photocontrol of nitric oxide production in cell culture using a caged isoform selective inhibitor, Bioorg. Med. Chem., 2005, 13, 47–57 CrossRef CAS PubMed.
- M. Goard, G. Aakalu, O. D. Fedoryak, C. Quinonez, J. St. Julien, S. J. Poteet, E. M. Schuman and T. M. Dore, Light-Mediated Inhibition of Protein Synthesis, Chem. Biol., 2005, 12, 685–693 CrossRef CAS PubMed.
- D. Abate-Pella, N. A. Zeliadt, J. D. Ochocki, J. K. Warmka, T. M. Dore, D. A. Blank, E. V. Wattenberg and M. D. Distefano, Photochemical Modulation of Ras-Mediated Signal Transduction Using Caged Farnesyltransferase Inhibitors: Activation by One- and Two-Photon Excitation, ChemBioChem, 2012, 13, 1009–1016 CrossRef CAS PubMed.
- S. Gug, S. Charon, A. Specht, K. Alarcon, D. Ogden, B. Zietz, J. Léonard, S. Haacke, F. Bolze, J.-F. Nicoud and M. Goeldner, Photolabile Glutamate Protecting Group with High One- and Two-Photon Uncaging Efficiencies, ChemBioChem, 2008, 9, 1303–1307 CrossRef CAS PubMed.
- D. Warther, F. Bolze, J. Léonard, S. Gug, A. Specht, D. Puliti, X.-H. Sun, P. Kessler, Y. Lutz, J.-L. Vonesch, B. Winsor, J.-F. Nicoud and M. Goeldner, Live-Cell One- and Two-Photon Uncaging of a Far-Red Emitting Acridinone Fluorophore, J. Am. Chem. Soc., 2010, 132, 2585–2590 CrossRef CAS PubMed.
- A. Leonidova, V. Pierroz, R. Rubbiani, Y. Lan, A. G. Schmitz, A. Kaech, R. K. O. Sigel, S. Ferrari and G. Gasser, Photo-Induced Uncaging of a Specific Re(I) Organometallic Complex in Living Cells, Chem. Sci., 2014, 4044–4056 RSC.
- N. Gagey, M. Emond, P. Neveu, C. Benbrahim, B. Goetz, I. Aujard, J.-B. Baudin and L. Jullien, Alcohol Uncaging with Fluorescence Reporting: Evaluation of o-Acetoxyphenyl Methyloxazolone Precursors, Org. Lett., 2008, 10, 2341–2344 CrossRef CAS PubMed.
- G. Papageorgiou, D. C. Ogden, A. Barth and J. E. T. Corrie, Photorelease of Carboxylic Acids from 1-Acyl-7-nitroindolines in Aqueous Solution: Rapid and Efficient Photorelease of L-Glutamate, J. Am. Chem. Soc., 1999, 121, 6503–6504 CrossRef CAS.
- S. C. Boca, M. Four, A. Bonne, B. van der Sanden, S. Astilean, P. L. Baldeck and G. Lemercier, An ethylene-glycol decorated ruthenium(ii) complex for two-photon photodynamic therapy, Chem. Commun., 2009, 4590–4592 RSC.
- S. Hatakeyama, K. Sugihara, T. K. Shibata, J. Nakayama, T. O. Akama, N. Tamura, S.-M. Wong, A. A. Bobkov, Y. Takano, C. Ohyama, M. Fukuda and M. N. Fukuda, Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 19587–19592 CrossRef CAS PubMed.
- G. M. Anstead, S. E. Sen and G. D. Prestwich, Evaluation of squalene analogs bearing photoreactive groups as inhibitors of squalene epoxidase and oxidosqualene cyclase, Bioorg. Chem., 1991, 19, 300–313 CrossRef CAS.
- P. Anstaett, A. Leonidova and G. Gasser, Caged Phosphate and the Slips and Misses in Determination of Quantum Yields for UV-A induced Photouncaging, ChemPhysChem, 2015, 16, 1857–1860 CrossRef CAS PubMed.
- J. E. T. Corrie, J. H. Kaplan, B. Forbush, D. C. Ogden and D. R. Trentham, Commentary on “Caged Phosphate and the Slips and Misses in Determination of Quantum Yields for Ultraviolet-A-Induced Photouncaging” by G. Gasser and Co-Workers, ChemPhysChem, 2015, 16, 1861–1862 CrossRef CAS PubMed.
- P. Anstaett, A. Leonidova, E. Janett, C. G. Bochet and G. Gasser, Reply to the Commentary by Trentham et al. on “Caged Phosphate and the Slips and Misses in Determination of Quantum Yields for Ultraviolet-A-Induced Photouncaging” by Gasser et al., ChemPhysChem, 2015, 16, 1863–1866 CrossRef CAS PubMed.
- T. Furuta, S. S.-H. Wang, J. L. Dantzker, T. M. Dore, W. J. Bybee, E. M. Callaway, W. Denk and R. Y. Tsien, Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1193–1200 CrossRef CAS.
- A. Specht, F. Bolze, J. F. Nicoud and M. Goeldner, in Chemical Neurobiology, ed. M. R. Banghart, Humana Press, New York, 2013, ch. 6, vol. 995, pp. 79–87 Search PubMed.
- R. R. Anderson and J. A. Parrish, The Optics of Human Skin, J. Investig. Dermatol., 1981, 77, 13–19 CAS.
- L. Donato, A. Mourot, C. M. Davenport, C. Herbivo, D. Warther, J. Léonard, F. Bolze, J.-F. Nicoud, R. H. Kramer, M. Goeldner and A. Specht, Water-Soluble, Donor–Acceptor Biphenyl Derivatives in the 2-(o-Nitrophenyl)propyl Series: Highly Efficient Two-Photon Uncaging of the Neurotransmitter γ-Aminobutyric Acid at λ = 800 nm, Angew. Chem., Int. Ed., 2012, 51, 1840–1843 CrossRef CAS PubMed.
- H. A. Collins, M. Khurana, E. H. Moriyama, A. Mariampillai, E. Dahlstedt, M. Balaz, M. K. Kuimova, M. Drobizhev, X. D. YangVictor, D. Phillips, A. Rebane, B. C. Wilson and H. L. Anderson, Blood-vessel closure using photosensitizers engineered for two-photon excitation, Nat. Photonics, 2008, 2, 420–424 CrossRef CAS PubMed.
- C. Bao, M. Jin, B. Li, Y. Xu, J. Jin and L. Zhu, Long conjugated 2-nitrobenzyl derivative caged anticancer prodrugs with visible light regulated release: preparation and functionalizations, Org. Biomol. Chem., 2012, 10, 5238–5244 CAS.
- M. Uhlén, L. Fagerberg, B. M. Hallström, C. Lindskog, P. Oksvold, A. Mardinoglu, Å. Sivertsson, C. Kampf, E. Sjöstedt, A. Asplund, I. Olsson, K. Edlund, E. Lundberg, S. Navani, C. A.-K. Szigyarto, J. Odeberg, D. Djureinovic, J. O. Takanen, S. Hober, T. Alm, P.-H. Edqvist, H. Berling, H. Tegel, J. Mulder, J. Rockberg, P. Nilsson, J. M. Schwenk, M. Hamsten, K. von Feilitzen, M. Forsberg, L. Persson, F. Johansson, M. Zwahlen, G. von Heijne, J. Nielsen and F. Pontén, Tissue-based map of the human proteome, Science, 2015, 347, 1260419 CrossRef PubMed.
- M. Uhlen, P. Oksvold, L. Fagerberg, E. Lundberg, K. Jonasson, M. Forsberg, M. Zwahlen, C. Kampf, K. Wester, S. Hober, H. Wernerus, L. Bjorling and F. Ponten, Towards a knowledge-based Human Protein Atlas, Nat. Biotechnol., 2010, 28, 1248–1250 CrossRef CAS PubMed.
- C. A. Puckett and J. K. Barton, Fluorescein Redirects a Ruthenium−Octaarginine Conjugate to the Nucleus, J. Am. Chem. Soc., 2009, 131, 8738–8739 CrossRef CAS PubMed.
- M. Triesscheijn, P. Baas, J. H. M. Schellens and F. A. Stewart, Photodynamic Therapy in Oncology, Oncologist, 2006, 11, 1034–1044 CrossRef CAS PubMed.
- A. Frei, R. Rubbiani, S. Tubafard, O. Blacque, P. Anstaett, A. Felgentraeger, T. Maisch, L. Spiccia and G. Gasser, Synthesis, Characterization and Biological Evaluati on of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy, J. Med. Chem., 2014, 57, 7280–7292 CrossRef CAS PubMed.
- M. R. Hamblin and T. Hasan, Photodynamic therapy: a new antimicrobial approach to infectious disease?, Photochem. Photobiol. Sci., 2004, 3, 436–450 CAS.
- J. A. Bertout, S. A. Patel and M. C. Simon, The impact of O2 availability on human cancer, Nat. Rev. Cancer, 2008, 8, 967–975 CrossRef CAS PubMed.
- P. Vaupel and A. Mayer, Hypoxia in cancer: significance and impact on clinical outcome, Cancer Metastasis Rev., 2007, 26, 225–239 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Instrumentation, methods, synthetic procedures and compound characterization, spectra. See DOI: 10.1039/c5pp00245a |
| This journal is © The Royal Society of Chemistry and Owner Societies 2015 |