On stoichiometry and intermixing at the spinel/perovskite interface in CoFe2O4/BaTiO3 thin films†
Vasiliki
Tileli
*a,
Martial
Duchamp
b,
Anna-Karin
Axelsson
ac,
Matjaz
Valant
ad,
Rafal E.
Dunin-Borkowski
b and
Neil McN.
Alford
a
aDepartment of Materials, Imperial College London, Exhibition Road, SW7 2AZ London, UK. E-mail: v.tileli@imperial.ac.uk
bErnst Ruska-Centre for Microscopy and Spectroscopy with Electrons and Peter Grünberg Institute, Forschungszentrum Jülich, 52425 Jülich, Germany
cSchool of Engineering, London South Bank University, 103 Borough Road, SE1 0AA London, UK
dMaterials Research Laboratory, University of Nova Gorica, Vipavska 13, Nova Gorica, SI-5000, Slovenia
First published on 10th November 2014
Abstract
The performance of complex oxide heterostructures depends primarily on the interfacial coupling of the two component structures. This interface character inherently varies with the synthesis method and conditions used since even small composition variations can alter the electronic, ferroelectric, or magnetic functional properties of the system. The focus of this article is placed on the interface character of a pulsed laser deposited CoFe2O4/BaTiO3 thin film. Using a range of state-of-the-art transmission electron microscopy methodologies, the roles of substrate morphology, interface stoichiometry, and cation intermixing are determined on the atomic level. The results reveal a surprisingly uneven BaTiO3 substrate surface formed after the film deposition and Fe atom incorporation in the top few monolayers inside the unit cell of the BaTiO3 crystal. Towards the CoFe2O4 side, a disordered region extending several nanometers from the interface was revealed and both Ba and Ti from the substrate were found to diffuse into the spinel layer. The analysis also shows that within this somehow incompatible composite interface, a different phase is formed corresponding to the compound Ba2Fe3Ti5O15, which belongs to the ilmenite crystal structure of FeTiO3 type. The results suggest a chemical activity between these two oxides, which could lead to the synthesis of complex engineered interfaces.
1 Introduction
Advanced heterostructured nanomaterials, such as embedded nanoparticles, heteroepitaxial composite layers, and even bio-hybrids with a functional response, are increasingly investigated for their expanded applications in electronics, materials science, catalysis, energy production, medicine, etc. In general, these functional nanostructures respond through a change of a state variable, which may be a change of charge or spin density, a spin or dipole orientation, an excited state, a mechanical deformation, a molecular arrangement, etc. In thin film layered composites, the interface becomes as important as the bulk of the individual layers. Sometimes it can even be considered as a completely new layer as both its physics and its chemistry deviate from either of the two components. The interfacial regions that are commonly found in oxide composites, are often formed from defects, strain, and interdiffusion; sometimes they can be of a completely different crystal structure or chemical composition. When the film thickness goes below about one hundred nanometers, it is very likely that up to about half of the film volume is structurally affected by the interface,1 which significantly changes its overall properties. In some cases, the new chemistry can be advantageous, like for the SrTiO3–LaAlO3 interface, which the interfacial bonding energies create a highly conductive 2D electron cloud. In some other cases, the interaction between the two layers impedes the desired functional properties, as recently reported for the LaCrO3/SrTiO3 system.2Spinel and perovskite structures are often combined into a multilayer composite in magnetoelectrics3,4 as some of the best magnetostrictive and piezoelectric oxides belong to these crystal groups. For such magnetoelectro composites, theoretical models claim that a perfect elastic interface between the magnetostrictive and piezoelectric layers provides optimal magnetoelectric coupling,5 assuming that the properties of the parent materials in the composite are not altered during processing. Consequently, any characteristics of the interfaces that will induce structural changes will reduce the magnetoelectric coupling. This is becoming increasingly important when the layer thickness is limited to nanometer scale. The composite CoFe2O4/BaTiO3 system has been studied in many different configurations, such as BaTiO3 nanopillars vertically clamped to a CoFe2O4 matrix,6 self-assembled nanostructures,7,8 two-phase ceramics,9 and planar (i.e. layered) heterostructures.10 It should be pointed out that, despite the vertical nanopillar structure being less clamped to the matrix, conductive interfaces can form and, thus, can electrically shut down the application.11 As a result, much attention is focused on the layered heterostructures where another problem is highlighted. The authors regularly observe a decrease in the magnetization of the CoFe2O4 layer after deposition and, consequently, a reduction in magnetoelectric coupling. Although the mechanism of the failure is still not fully understood, it is evident that the problems originate from the interfacial characteristics of the two oxide layers.
To explain the atomistic origin of this problem, it is important to understand what drives the electric and magnetic order in each individual crystal structure. In tetragonal room-temperature BaTiO3 with a P4mm cell, Ti atoms are displaced along the c-axis and the cell is therefore elongated. This elongation is discrete and its high polar state originates from the mass and charge of the Ba atom. This in turn means that the ferroelectric and piezoelectric properties of BaTiO3 are very sensitive to the average relative displacement along the polar c-axis cell, which moreover means that impurities and strain would dramatically change these properties. On the other hand, the magnetostrictive oxide, i.e. CoFe2O4, has an inverse spinel structure with formula B(AB)O4. In an ideal inverse spinel, Co2+ ions occupy the octahedral sites and Fe3+ ions are equally divided among the remaining octahedral sites and the tetrahedral site. It has a cubic lattice parameter of 8.38 Å, which corresponds to a ∼7% lattice mismatch with the (001) BaTiO3 growth template (a(CoFe2O4) = 2a(BaTiO3)). Only an eighth of the tetrahedral sites and half of the octahedral sites are occupied in this spinel. This large fraction of empty interstitial sites creates an open structure that is susceptible to strain effects and readily permits cation migration within the spinel. In reality, the cation occupancy in the CoFe2O4 spinel structure deviates from the ideal distribution, which directly affects the magnetic properties due to antiferromagnetic ordering in the two spin sub-lattices.
Typically, the synthesis of spinel-perovskite thin layers proceeds either by pulsed laser deposition (PLD) or by molecular beam epitaxy (MBE). The preferred method is PLD due to its high throughput, ease of use and inexpensive nature compared to MBE. However, control of the composition of heterostructures remains a tantalizing issue for both techniques but, more so, for the highly, energetic PLD.2 Consequently, the functional properties of complex oxides depend strongly on all processing factors during a typical vacuum film deposition such as atmosphere, pressure, and temperature.
The objective of this paper is to get an insight into the atomic arrangement of the interface of the PLD grown CoFe2O4–BaTiO3 magnetoelectric thin film system by means of advanced sub-nanometer transmission electron microscopy techniques. We discuss the role of the BaTiO3 substrate morphology and polarity on the structural properties of the interface. Furthermore, we report on chemical interdiffusion, cation intermixing, and valence state and coordination instabilities occurring during CoFe2O4 film deposition and relate them to the observed deterioration of the magnetoelectric properties. This is the first report to suggest another compound forming at the interface of stoichiometry Ba2Fe3Ti5O15, which is a reduced A-site Ba doped ilmenite of FeTiO3 type, further corroborating the chemical complexity of the heterostructure.
2 Experimental details
CoFe2O4 films were grown by pulsed laser deposition (PLD) at 550 °C on (001) BaTiO3 single crystal substrate. Details of the deposition have been reported elsewhere.12 Film thicknesses ranged from 13–100 nm. All films show similar interfacial characteristics and, for the work here, the sample of 75 nm thick film was analyzed. Cross-sectional specimens were prepared by focused ion beam milling and final thinning was performed with low energy Ar ions (0.5 eV) using a Fischione NanoMill system. Structural investigations were performed using an FEI Titan transmission electron microscope (TEM) equipped with a spherical aberration corrector at the image plane (London, UK). For all TEM micrographs, the corrector was tuned to −13 μm. High angle annular dark field (HAADF) scanning transmission electron micrographs (STEM) and chemical investigations were carried out using an FEI Titan double corrected TEM equipped with a Gatan Quantum electron energy-loss (EEL) spectrometer (Jülich, Germany) with an electron energy of 200 keV. The STEM resolution was of 0.8 Å. Electron energy-loss spectroscopy (EELS) data were acquired with an energy resolution of 700 meV and an energy dispersion of 0.2 eV per channel. The spectrum image (SI) analysed here was 182 × 22 pixels and it was acquired with 0.51 Å step size. It is noted that multiple EEL SIs were acquired and no significant compositional variations were detected across the interface. The EEL spectrum image analyzed below was acquired at a collection semi-angle of 40 mrad and a convergence semi-angle of 20 mrad. To reduce noise in the HAADF STEM images from Fig. 1(d) and Fig. 5(a) below, a gaussian low pass filter of 0.015 nm σ was applied. Simulations of atomic positions were performed using Vesta software13 and STEM images were calculated using Dr Probe software.14 Finally, we note that the projected final cross-section thickness of the specimen analysed was ∼10 nm for the TEM measurements and on the order of 50 nm for the STEM/EELS analysis.3 Results and discussion
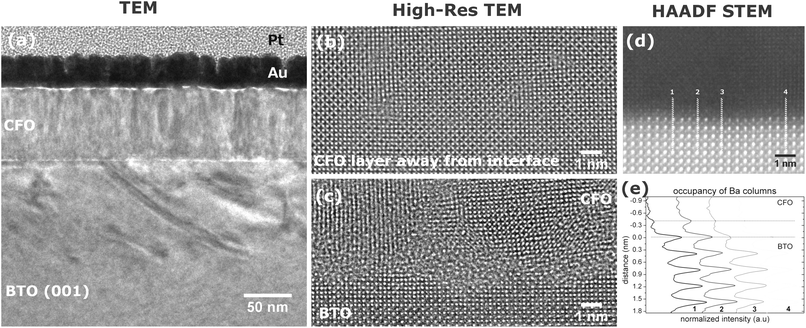
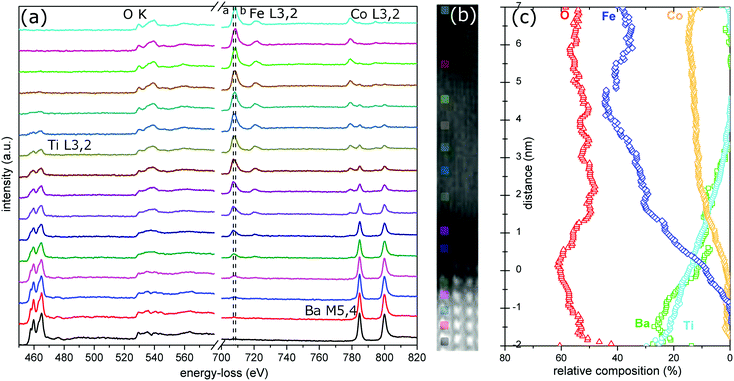
The local structure of the system CoFe2O4/BaTiO3 was evaluated by TEM, as shown in Fig. 1(a–c), and by STEM, as shown in Fig. 1(d and e). The impact of the growth of the film on the highly polar BaTiO3 substrate is demonstrated well in Fig. 1(a) where contrast variations due to strain effects are extended over 100 nm into the substrate. The film is epitaxially grown (also revealed by XRD1) and it proceeds with a columnar character. The strain induced by the 7% lattice mismatch is compensated by minor rotations of unit cells and dislocations. A low density of dislocations is integrated throughout the CoFe2O4 film, as shown in Fig. 1(b). High-resolution (High-Res) TEM is sensitive to the phase of the exit surface electron wave. In this case (Fig. 1(c)), the interface appears as a low crystallinity layer with a varying thickness profile. This highly defective interface is adopted to compensate for the enhanced strain of the surface layer induced by the intrinsic strong surface polarization fields, as previously argued.15 In addition, BaTiO3 develops a surface step structure during film deposition. Evidence of atomic intermixing in the last 3 unit cells of the substrate can be seen on the left side of the Fig. 1(d). These issues are expected to further introduce hindrances on the film growth. STEM is more sensitive to the atomic number of the chemical element present in the structure and, under the HAADF imaging mode, diffraction and strain effects are minimized. In the HAADF STEM image of the interface (Fig. 1(d)), the role of the imperfect substrate surface is augmented. For a qualitative evaluation of the occupancy of Ba columns near the interface, histograms taken from across the interface are plotted in Fig. 1(e). The comparison indicates atomically imperfect interfacial planes at the substrate surface where the occupancy of Ba varies significantly along the same unit cell and, in cases, it is half the value of the bulk. Such disorder of the surface structure of the substrate suggests a variation of the bound surface charge density that is responsible for the ferroelectric polarization and, thus, it reflects the quality of the magnetoelectric coupling of the composite.The local structural environment is related to the stoichiometry and the chemistry at the interface by quantitative analysis of core-loss EEL spectra. Fig. 2 shows typical atomically-resolved EEL spectra and a corresponding annular dark field (ADF) STEM image extending from the pure perovskite substrate into the fully spinel film. The raw EEL spectra demonstrate high mobility of Fe, Ba, and Ti across the interface. Fe seems to be incorporated into the BaTiO3 unit cells near the interface. According to previous studies in ceramics,16 Fe can substitute both A and B sites in BaTiO3 and, in both cases, the end result is a decrease of the ferroelectric polarization of the perovskite. Accordingly, in the case of this thin film structure, the interfacial strain can induce further decrease of the surface polarization. In the CoFe2O4 film, the EEL spectra indicate Ba and Ti diffusion by ∼3 nm from the interface. Substitution of diamagnetic ions (Ba, Ti) for paramagnetic ions (Co, Fe) in the spinel can reduce the magnetization and Néel temperature and eventually lead to a paramagnetic room-temperature system.
The transition between the two structures can be effectively monitored by following the oxygen K edge, which is shown alongside all major peaks in Fig. 2(a). The O K fine structure in the substrate, which is shown in the lowest spectrum and it is better monitored in Fig. 3(b), contains four characteristic peaks (a–d) reflecting the perovskite lattice.17–19 In detail, the first peak (a) is related to the hybridization of the titanium 3d orbitals and the oxygen 2p orbitals, the second peak (b) is dominated by Ba–O interactions, whereas the third (c) and fourth (d) peaks arise from scattering at the nearest oxygen shell.20 On the other side of the interface, in the upper spectrum, the O energy-loss near edge fine structure (ELNES) for CoFe2O4 shows inverse spinel characteristics in accordance with previous studies.21,22 In particular, transitions to states created by the hybridisation of oxygen 2p take place with three types of 3d orbitals; the first peak (a) is attributed to a tetrahedrally co-ordinated iron(III) orbital, whereas the broad peak (b) arises due to octahedrally co-ordinated orbitals for both iron(III) and cobalt(II) ions.
 | ||
| Fig. 3 ELNES for the core loss edges of (a) Ti L3,2, (b) O K, (c) Fe L3,2 and (d) Ba M5,4 and Co L3,2 showing the evolution of the structure from BaTiO3 towards the interface at position 0 (nm), at position 2 (nm), and within the fully inverse CoFe2O4 spinel film. For the positions of the distances along the interface, refer to the HAADF image of Fig. 2(b). The characteristic peak positions for each ion are indicated for clarity. | ||
The interface between the two structures extends over several nanometers and the chemical transition, inferred from the EEL spectra, is much wider than the dark interfacial contrast visible in STEM images, and it is not confined to a monolayer as reported for the BaTiO3/Fe system recently.23 The O K edge fine structure becomes broad at position 0 (reflecting the beginning of the interface), when a significant amount of Fe is incorporated into the BaTiO3 unit cell. Multiple linear least squares (MLLS) analysis of the interface O K edge with spectra from the outer “bulk-like” BaTiO3 and CoFe2O4 fine structures suggests that the interface is not composed of the two distinct structures but consists of a different phase (see Fig. S2†). In order to obtain information about this interfacial phase, quantification of the EEL spectra was performed. The relative composition of the different elements was established by carrying out quantitative analysis by integrating the intensities of the ionization edges related to the partial ionization cross-sections.24 For example, for the Ba M edge and the Ti L edge the ratio of the absolute numbers N of atoms per unit area in the specimen can be expressed in the form:
 | (1) |
Values of the ionization cross-sections (σ) of Ba M, Ti L, Fe L, and Co L edges were calculated using (i) standard spectra taken from bulk BaTiO3 and “bulk-like” CoFe2O4 taken several nanometers away from interface and (ii) the calculated O K edge cross-section using Hartree–Slater methods implemented in DigitalMicrograph software (assumed to be a good approximation for K edges of light elements). These corrected ionization cross-sections were used to quantify the amount of each element and plot the calculated quantification profiles versus depth, which are shown in Fig. 2(c). The calculated cross-sections and the integration ranges are shown in Table S1.† The average error in the analysis is estimated to ∼10%, which includes contributions from the background subtraction, integration window widths, and cross-section calculations.24
At the interface (position 0 at Fig. 2(c)) the relative extracted composition corresponds to a compound of stoichiometry Ba2Fe3Ti5O15. This compound can be reduced to the ilmenite structure assuming that Ba2+ ions occupy a proportion of the Fe sites, which should be of 2+ valency to ensure charge neutrality. Indeed, the shift of the Fe L3 edge at the interface towards lower energy losses is already visible in the raw spectra (Fig. 2(a)). Ilmenite-derived materials have been reported to show A-site-driven geometric ferroelectricity.25 Two nanometers away from the interface, at position 2, the quantitative analysis suggests a structure of BaTiCo2Fe4O10. The spectra shown in Fig. 3 suggest incorporation of Ba2+ ions on the Co sites and Ti4+ ions on the Fe sites. Both valence states of iron are found. When Ti is incorporated into the lattice, the Fe oxidation state is reduced. The O K edge indicates a spinel-like character of the structure, with distinct a and b peaks. It is noted that inspection of the Ti L3 edge shows similar features across the interface, with no indication of a possible reduction of the valence state of Ti from 4+ to the magnetic 3+ state (although its determination from EELS results is challenging depending on the relative proportion of the two oxidation states).
To evaluate the predicted structures, Fe valence maps were calculated using a multiple linear least squares algorithm in DigitalMicrograph software. Standard spectra (shown in Fig. S3†) were used for octahedrally coordinated Oh Fe2+ (ref 26) and for tetrahedrally Td and octahedrally coordinated Oh Fe3+ (ref. 27). The profiles of the fitted coefficients for each valence and coordination state of Fe and the corresponding maps are shown in Fig. 4. The Oh Fe2+ signal dominates the interface, it peaks at position 2 nm and then it linearly decreases further away from the interface. CoFe2O4 standard spectra taken 17 nm from the interface show a diminishing amount of 2+ oxidation state of Fe. The change of the coordination of the Fe 3+ oxidation state is related to the evolution towards the fully spinel structure. The interface, position 0, is dominated by Fe 2+ oxidation state and any spinel inversion measurement is not possible. However, already from Fig. 4, the ratio FeOh3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeTd3+ at position 7(nm) is seen to be equal to 2
FeTd3+ at position 7(nm) is seen to be equal to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, corresponding to a fully inverse spinel structure. It is noted that the degree of inversion of the spinel CoFe2O4 has been related to the magnetic moment previously both experimentally28 and theoretically.29 All previous studies suggest that the total magnetic moment decreases with decreasing inversion, inducing an exchange mechanism of Co ions on octahedral sites with tetrahedrally coordinated Fe ions. Therefore, the reduced magnetic moment in such thin films (Fig. S1†) is attributed to the fact that a significant part of the film right at the interface is not fully inversed.
1, corresponding to a fully inverse spinel structure. It is noted that the degree of inversion of the spinel CoFe2O4 has been related to the magnetic moment previously both experimentally28 and theoretically.29 All previous studies suggest that the total magnetic moment decreases with decreasing inversion, inducing an exchange mechanism of Co ions on octahedral sites with tetrahedrally coordinated Fe ions. Therefore, the reduced magnetic moment in such thin films (Fig. S1†) is attributed to the fact that a significant part of the film right at the interface is not fully inversed.
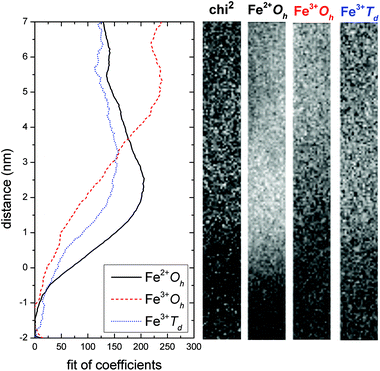 | ||
| Fig. 4 Multiple linear least squares (MLLS) fit of coefficients along the spectrum image in Fig. 2. The fit was computed using standard spectra for the octahedrally Oh coordinated Fe2+ and the tetrahedrally Td and octahedrally Oh coordinated Fe3+. The maps of each of the components indicate a high concentration of the 2+ oxidation state of Fe at the interface. | ||
In an attempt to relate the chemical results to the structural character of the CoFe2O4–BaTiO3 interface, a predicted model structure of a perfect interface is super-imposed on a representative HAADF STEM image, seen in Fig. 5(a). For the atomic layer stacking at the interface, a terminating layer of TiO2 was considered on the substrate and an Fe–O terminating layer on the CoFe2O4 (001), which reflects the Fe-rich interface, as deducted from the EELS results. This model is clearly not plausible since the regular spinel structure is not in contact with the substrate surface. Based on the chemical analysis, for the intermediate layer (about 2 nm thick), we consider a supercell along
4 Conclusions
Our findings reveal for the first time on an atom-by-atom scale the chemical complexity of the heteroepitaxy between CoFe2O4 and BaTiO3 along the (001) crystallographic direction. The inherent polarity of the substrate results in surface strain at both sides of the interface. This strain propagates for about 100 nm into the bulk substrate whereas at the CoFe2O4 side it is accommodated by defects and dislocations. The adopted, highly disordered interfacial structure is ∼2 nm thick and it is non-stoichiometric. In addition, the Fe is reduced to the 2+ oxidation state and the spinel full inverses only after ∼5 nm into the film. Quantitative analysis uncovered an ilmenite-like structure as the immediate interfacial layer. The results provide structural and chemical evidence of the incompatibility of the ferroelectric BaTiO3 with the ferrimagnetic CoFe2O4. Ultimately, the failure to control the quality at the interface impedes the elastic strain effects required for the strong magnetoelectric coupling of the composite system. It is noted that although the above results were acquired from a thin film grown under specific conditions, it is expected that, at some degree, the stoichiometry and intermixing of the two oxides will prevail for highly energetic techniques such as pulsed laser deposition.Acknowledgements
The authors are grateful to Dr Giuseppe Mallia for fruitful discussions concerning the atomic modeling of the interface. The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant agreement no [301838]. The authors acknowledge further financial support from the EU under the Seventh Framework Programme under grant Agreement 312483-ESTEEM2 (Integrated Infrastructure Initiative I3).References
- A.-K. Axelsson, F. Aguesse, V. Tileli, M. Valant and N. M. Alford, J. Alloys Compoud., 2013, 578, 286–291 CrossRef CAS PubMed.
- L. Qiao, K. H. L. Zhang, M. E. Bowden, T. Varga, V. Shutthanandan, R. Colby, Y. Du, B. Kabius, P. V. Sushko, M. D. Biegalski and S. A. Chambers, Adv. Funct. Mater., 2013, 23, 2953–2963 CrossRef CAS.
- M. Fiebig, J. Phys. D: Appl. Phys., 2005, 38, R123–R152 CrossRef CAS.
- W. Eerenstein, N. D. Mathur and J. F. Scott, Nature, 2006, 442, 759–765 CrossRef CAS PubMed.
- M. I. Bichurin, V. M. Petrov and G. Srinivasan, J. Appl. Phys., 2002, 92, 7681–7683 CrossRef CAS PubMed.
- H. Zheng, J. Wang, S. E. Lofland, Z. Ma, L. Mohaddes-Ardabili, T. Zhao, L. Salamanca-Riba, S. R. Shinde, S. B. Ogale, F. Bai, D. Viehland, Y. Jia, D. G. Schlom, M. Wuttig, A. Roytburd and R. Ramesh, Science, 2004, 303, 661–663 CrossRef CAS PubMed.
- H. Zheng, J. Wang, L. Mohaddes-Ardabili, M. Wuttig, L. Salamanca-Riba, D. G. Schlom and R. Ramesh, Appl. Phys. Lett., 2004, 85, 2035–2037 CrossRef CAS PubMed.
- D. H. Kim, N. M. Aimon, X. Sun and C. A. Ross, Adv. Funct. Mater., 2014, 24, 2334–2342 CrossRef CAS.
- J. Van Den Boomgaard, A. M. J. G. Van Run and J. V. Suchtelen, Ferroelectrics, 1976, 10, 295–298 CrossRef CAS.
- R. V. Chopdekar and Y. Suzuki, Appl. Phys. Lett., 2006, 89, 182506 CrossRef PubMed.
- Y.-H. Hsieh, J.-M. Liou, B.-C. Huang, C.-W. Liang, Q. He, Q. Zhan, Y.-P. Chiu, Y.-C. Chen and Y.-H. Chu, Adv. Mater., 2012, 24, 4564–4568 CrossRef CAS PubMed.
- F. Aguesse, A.-K. Axelsson, M. Valant and N. M. Alford, Scr. Mater., 2012, 67, 249–252 CrossRef CAS PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2008, 41, 653–658 CrossRef CAS.
- J. Barthel and L. Houben, Dr. Probe, High-resolution (S)TEM image simulation software, 2013, http://www.er-c.org/barthel/drprobe/index.html.
- F. L. English, J. Appl. Phys., 1968, 39, 3231–3236 CrossRef CAS PubMed.
- N. Maikhuri, A. K. Panwar and A. K. Jha, J. Appl. Phys., 2013, 113 CrossRef CAS PubMed , 17D915.
- N. Browning, D. Wallis, P. Nellist and S. Pennycook, Micron, 1997, 28, 333–348 CrossRef CAS.
- F. M. F. de Groot, M. Grioni, J. C. Fuggle, J. Ghijsen, G. A. Sawatzky and H. Petersen, Phys. Rev. B: Condens. Matter, 1989, 40, 5715–5723 CrossRef CAS.
- M. Bugnet, G. Radtke and G. A. Botton, Phys. Rev. B: Condens. Matter, 2013, 88, 201107 CrossRef.
- J. Zhang, A. Visinoiu, F. Heyroth, F. Syrowatka, M. Alexe, D. Hesse and H. S. Leipner, Phys. Rev. B: Condens. Matter, 2005, 71, 064108 CrossRef.
- W. F. Pong, M. H. Su, M.-H. Tsai, H. H. Hsieh, J. Y. Pieh, Y. K. Chang, K. C. Kuo, P. K. Tseng, J. F. Lee, S. C. Chung, C. I. Chen, K. L. Tsang and C. T. Chen, Phys. Rev. B: Condens. Matter, 1996, 54, 16641–16645 CrossRef CAS.
- S. Gautam, S. Muthurani, M. Balaji, P. Thakur, D. P. Padiyan, K. H. Chae, S. S. Kim and K. Asokan, J. Nanosci. Nanotechnol., 2011, 11, 386–390 CrossRef CAS PubMed.
- L. Bocher, A. Gloter, A. Crassous, V. Garcia, K. March, A. Zobelli, S. Valencia, S. Enouz-Vedrenne, X. Moya, N. D. Marthur, C. Deranlot, S. Fusil, K. Bouzehouane, M. Bibes, A. Barthelemy, C. Colliex and O. Stephan, Nano Lett., 2012, 12, 376–382 CrossRef CAS PubMed.
- R. F. Egerton, Electron energy-loss spectroscopy in the electron microscope, Plenum Press, New York, NY, 1986 Search PubMed.
- N. A. Benedek and C. J. Fennie, J. Phys. Chem. C, 2013, 117, 13339–13349 CAS.
- G. Radtke, S. Lazar and G. A. Botton, Phys. Rev. B: Condens. Matter, 2006, 74, 155117 CrossRef.
- S. Turner, J. Verbeeck, F. Ramezanipour, J. E. Greedan, G. Van Tendeloo and G. A. Botton, Chem. Mater., 2012, 24, 1904–1909 CrossRef CAS.
- G. A. Sawatzky, F. VAN DER Woude and A. H. Morrish, J. Appl. Phys., 1968, 39, 1204–1205 CrossRef CAS PubMed.
- Y. H. Hou, Y. J. Zhao, Z. W. Liu, H. Y. Yu, X. C. Zhong, W. Q. Qiu, D. C. Zeng and L. S. Wen, J. Phys. D: Appl. Phys., 2010, 43, 445003 CrossRef.
- T. Varga, A. Kumar, E. Vlahos, S. Denev, M. Park, S. Hong, T. Sanehira, Y. Wang, C. J. Fennie, S. K. Streiffer, X. Ke, P. Schiffer, V. Gopalan and J. F. Mitchell, Phys. Rev. Lett., 2009, 103, 047601 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Magnetic moment data of the structure, linear decomposition graph of the interface layer in its constituent components of CoFe2O4 and BaTiO3, reference Fe L3,2 EEL data used for MLLS analysis of the Fe oxidation and coordination variation, and a table detailing the partial ionization cross-sections used for quantitative MLLS analysis. See DOI: 10.1039/c4nr04339a |
| This journal is © The Royal Society of Chemistry 2015 |