High interfacial storage capability of porous NiMn2O4/C hierarchical tremella-like nanostructures as the lithium ion battery anode†
Wenpei
Kang
a,
Yongbing
Tang
*ab,
Wenyue
Li
ab,
Xia
Yang
a,
Hongtao
Xue
a,
Qingdan
Yang
a and
Chun-Sing
Lee
*a
aDepartment of Physics and Materials Science and Center of Super-Diamond and Advanced Films (COSDAF), City University of Hong Kong, Hong Kong SAR, People's Republic of China. E-mail: tangyb@siat.ac.cn; apcslee@cityu.edu.hk; Tel: +852-34427826
bFunctional Thin Films Research Center, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, People's Republic of China
First published on 15th October 2014
Abstract
Porous hierarchical NiMn2O4/C tremella-like nanostructures are obtained through a simple solvothermal and calcination method. As the anode of lithium ion batteries (LIBs), porous NiMn2O4/C nanostructures exhibit a superior specific capacity and an excellent long-term cycling performance even at a high current density. The discharge capacity can stabilize at 2130 mA h g−1 within 350 cycles at a current density of 1000 mA g−1. After a long-term cycling of 1500 cycles, the capacity is still as high as 1773 mA h g−1 at a high current density of 4000 mA g−1, which is almost five times higher than the theoretical capacity of graphite. The porous NiMn2O4/C hierarchical nanostructure provides sufficient contact with the electrolyte and fast three-dimensional Li+ diffusion channels, and dramatically improves the capacity of NiMn2O4/C via interfacial storage.
1. Introduction
Hierarchical nanostructures or superstructures have attracted extensive attention owing to the synergistic effects of their nanometer-sized building blocks and overall micrometer-sized structures.1–6 These hierarchical structures not only provide characteristic size effects, but also give desirable mechanical strength, facile ion transportation and easy recycling. They are considered promising candidates for applications in high performance energy conversion and storage devices,7–12 catalysts,11–13 and adsorbents.14,15 These hierarchical structures are also important as potential electrode materials for lithium ion batteries (LIBs) and exhibit superior electrochemical performances because of their unique properties compared with their bulk counterparts.16–19As an important spinel binary metal oxide, NiMn2O4 has been applied in many fields, such as magnetism,20,21 catalysis,22 negative temperature coefficient thermistors,23 sensors,24 supercapacitor, etc.25,26 NiMn2O4 nanostructures have been synthesized by various methods including solid-state reaction,27 co-precipitation,28 sol–gel,29 and microemulsion-mediated routes.21 Additionally, a precursor method has been widely used to prepare binary metal oxides such as ZnMn2O4, CoMn2O4, NiMn2O4, CuMn2O4, etc.30–35 Porous NiMn2O4 nanostructures have been successfully prepared using an oxalate precursor,21,25 and exhibit an improved electrochemical performance due to their porous characteristic. As anode candidates of LIBs, nanostructured transition metal oxides have attracted much attention for their merits of a high surface-to-volume ratio and a short path length for Li-ion diffusion.36–41 Particularly, binary metal oxides have been among the most widely investigated alternative anode materials because of their much higher specific capacities (∼1000 mA h g−1) compared to that of conventional graphite (372 mA h g−1).42–45 Among these oxides, Mn-based oxides AMn2O4 (M = Co, Zn, Mn) exhibit excellent anodic performance. Lou's group reported a capacity of 750 mA h g−1 in ZnMn2O4 after 120 cycles30 and a capacity of 624 mA h g−1 in CoMn2O4 after 50 cycles.31 The CoMn2O4 anode was also reported to have a capacity of 706 mA h g−1 after 25 cycles by Xiong's group.17
Considering that nanostructured nickel-based oxides are also electrochemically active and can contribute to the lithium storage capability, we studied the electrochemical performances of Ni-containing Mn-based oxide as an anode material for LIBs. Recent studies showed that hierarchical geometries, especially porous nanostructures, can significantly increase the capacities of oxide anode materials due to interfacial lithium storage as reported by Maier et al. and re-oxidization to a higher oxidation state upon cycling.46–51 Herein, we report a porous NiMn2O4/C hierarchical tremella-like nanostructure assembled with nanoparticles via a simple solvothermal and calcination method for LIB application. This porous hierarchical nanostructure effectively facilitates Li+ ion transport by decreasing the diffusion distance and accommodates volume changes during charging/discharging processes. As the anode of LIBs, this tremella-like nanostructure exhibits a stable capacity of ∼2130 mA h g−1 at a high current density of 1000 mA g−1 after 350 cycles. Moreover, it still retains a capacity of ∼1773 mA h g−1 even at a higher current density of 4000 mA g−1 after 1500 cycles, indicating its potential application for LIBs with long cycle life and high power density.
2. Experimental
2.1 Preparation of NiMn2O4/C
All reagents were used as received. Typically, 0.5 mmol of Ni(CH3COO)2·4H2O, 1.0 mmol of Mn(CH3COO)2·2H2O and 3 mmol of hexamethylenetetramine were dissolved in a mixed solvent of 5.0 mL water and 25.0 mL triethylene glycol (TEG). The above solution was then transferred to a Teflon-lined stainless steel autoclave and held at 180 °C for 16 h. After naturally cooling to room temperature, the precursor precipitate was collected by centrifugation, washed several times with deionized water and ethanol, and vacuum dried at 60 °C for 12 h. The obtained precursor was mixed with polyacrylonitrile (PAN) at a weight ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the solvent N,N-dimethylformamide and then dried at 150 °C on a hot plate. To obtain the final products of NiMn2O4/C, the above mixture was first calcinated at 250 °C in air for 3 h and then at 600 °C in N2 for 3 h.
1 in the solvent N,N-dimethylformamide and then dried at 150 °C on a hot plate. To obtain the final products of NiMn2O4/C, the above mixture was first calcinated at 250 °C in air for 3 h and then at 600 °C in N2 for 3 h.
2.2 Materials characterization
X-ray diffraction (XRD) measurements were carried out on a Siemens D-500 diffractometer using Cu Kα radiation. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were respectively carried out with a Philips XL30 FEG SEM and a Philips FEG TEM CM200 (operated at 200 kV). X-ray photoelectron spectroscopy (XPS) analysis was performed on a VG ESCALAB 220i-XL UHV surface analysis system equipped with a monochromatic Al Kα X-ray source (1486.6 eV). Thermogravimetric analyzer (TGA) measurements were performed under an air atmosphere at a heating rate of 10 °C min−1 from room temperature to 600 °C. Fourier transform infrared spectroscopy (FTIR) measurements were performed on a VERTEX-70 spectrometer using the KBr tablet method.2.3 Electrochemical measurements
Working electrodes were prepared by mixing NiMn2O4/C with acetylene black and sodium alginate at a weight ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and with a total weight loading of 1.2 ± 0.3 mg cm−2. LiPF6 solution (1 mol L−1) in an ethylene carbonate and dimethyl carbonate mixture (1
2 and with a total weight loading of 1.2 ± 0.3 mg cm−2. LiPF6 solution (1 mol L−1) in an ethylene carbonate and dimethyl carbonate mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as the electrolyte. Galvanostatic cycling tests were performed using a Macco Instruments system. Cyclic voltammetry (CV) measurements were carried out over a potential window of 0.01–3.0 V with an electrochemical workstation (CHI 660D). Electrochemical impedance spectroscopy (EIS) was carried out on a ZAHNER-elektrik IM6 over a frequency range of 100 kHz to 10 mHz.
1, v/v) was used as the electrolyte. Galvanostatic cycling tests were performed using a Macco Instruments system. Cyclic voltammetry (CV) measurements were carried out over a potential window of 0.01–3.0 V with an electrochemical workstation (CHI 660D). Electrochemical impedance spectroscopy (EIS) was carried out on a ZAHNER-elektrik IM6 over a frequency range of 100 kHz to 10 mHz.
3. Results and discussion
A tremella-like carbonate precursor was obtained with the described solvothermal process. To obtain the desired bimetallic salt, instead of a mixture of the two monometallic salts, the processing conditions have to be optimized such that the solubility of the desired bimetallic salt is lower than that of individual monometallic salts.21 To realize this, polyol is usually used in the solvothermal reaction.52–54 In our studies, the mixture of TEG and water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as the solvent for the preparation of the binary metal carbonate precursor with tremella-like nanostructures. A series of control experiments has also been carried out to emphasize the importance of solvents (Fig. S1 and S2†) and the raw materials of acetate (Fig. S3†) for the formation of the carbonate precursor with the tremella-like nanostructures.
1, v/v) was used as the solvent for the preparation of the binary metal carbonate precursor with tremella-like nanostructures. A series of control experiments has also been carried out to emphasize the importance of solvents (Fig. S1 and S2†) and the raw materials of acetate (Fig. S3†) for the formation of the carbonate precursor with the tremella-like nanostructures.
The bottom curve of Fig. 1a shows XRD patterns of the as-prepared precursor. All the peaks can be well indexed based on the standard XRD patterns of MnCO3 (JCPDS no. 86-0173) and NiCO3 (JCPDS no. 78-0210). Together with the energy dispersive spectroscopy (EDS) analysis (Fig. S4†), the precursor can be confirmed as Ni1/3Mn2/3CO3. After mixing with PAN and calcinating at 600 °C, all the diffraction peaks in the XRD patterns (the top curve in Fig. 1a) of the obtained sample can be assigned to well-crystallized cubic spinel NiMn2O4 (JCPDS no. 84-0542). The oxidation state of the corresponding transition metal ions in the obtained sample was further investigated by X-ray photoelectron spectroscopy (XPS). A survey spectrum (Fig. 1b) shows the presence of Ni, Mn, and O as well as C and there is no other impurity. A Mn 2p core level spectrum (Fig. 1c) shows two major peaks with binding energies of 641.7 and 653.2 eV, assigned to the Mn 2p3/2 and Mn 2p1/2 peaks, respectively.17 After the refined fitting, the spectrum is composed of four peaks. Those with binding energies of 641.8 eV and 653.3 eV are ascribed to Mn3+. Another two peaks at 640.7 eV and 652.3 eV are ascribed to Mn2+. Similarly, the Ni 2p spectrum (Fig. 1d) shows two spin–orbit doublets characteristic of Ni2+ and Ni3+ states and shake-up peaks at around 861.2 and 879.7 eV at the high binding energy side of the Ni 2p3/2 and Ni 2p1/2 edges.55,56 The fitted peaks at 854.5 and 872.1 eV are attributed to Ni2+, while the other peaks at 855.8 and 874.0 eV are related to Ni3+. According to the XPS analyses, the couples of Mn3+/Mn2+ and Ni3+/Ni2+ are coexisting in the spinel NiMn2O4 nanostructures. The atomic ratio of Ni and Mn is ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 based on the areas of their corresponding XPS peaks.
2 based on the areas of their corresponding XPS peaks.
 | ||
| Fig. 1 (a) XRD patterns of NiMn2O4/C and its precursor Ni1/3Mn2/3CO3; XPS spectra for the as-prepared NiMn2O4 nanostructure: (b) a survey spectrum, (c) a Mn 2p and (d) a Ni 2p core level spectra. | ||
Fig. 2a displays SEM images of the as-prepared Ni1/3Mn2/3CO3 precursor. It can be seen that the precursor has a tremella-like hierarchical morphology assembled by many nanoplatelets as shown in the inset of Fig. 2a. It was found that the morphology of the obtained precursor depends strongly on the reaction solvent. Only irregular aggregates composed of small particles can be formed if water is used as the solvent (Fig. S1†). Meanwhile, if the solvent is pure TEG, the obtained precursor shows non-uniform feather-like structures (Fig. S2a and 2b†). Star-like structures can be obtained in a solvent of TEG and water with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S2c and 2d†). TEM observations indicated that the carbonate precursor can be well dispersed (Fig. 2b), and many nanoplatelets were integrated into a tremella-like hierarchical structure (inset of Fig. 2b).
1 (Fig. S2c and 2d†). TEM observations indicated that the carbonate precursor can be well dispersed (Fig. 2b), and many nanoplatelets were integrated into a tremella-like hierarchical structure (inset of Fig. 2b).
 | ||
| Fig. 2 (a) SEM and (b) TEM images of the Ni1/3Mn2/3CO3 precursor. The insets are the corresponding magnified images. | ||
To gain more insight into the actual evolution process of the tremella-like precursor structure, a series of time-dependent experiments were performed, and intermediate products were obtained at different reaction intervals (Fig. S5†). The XRD patterns indicate that the carbonate precursor forms from the initial reaction stage and the crystallinity increases with the reaction time. The sequential SEM images reveal a morphological evolution from random particles to the coexistence of particles and flakes, then the coexistence of particles, flakes and flake aggregates, and finally to the 3D hierarchical tremella-like nanostructures. We propose a precipitation–dissolution–recrystallization-oriented aggregation mechanism to explain the formation of the Ni1/3Mn2/3CO3 tremella-like structure. Finally, after calcination, porous NiMn2O4/C nanostructures can be obtained. Preparation of the tremella-like hierarchical structure is schematically illustrated in Fig. 3.
 | ||
| Fig. 3 Schematic diagram for the formation of Ni1/3Mn2/3CO3 tremella-like structures and porous NiMn2O4/C nanostructures. | ||
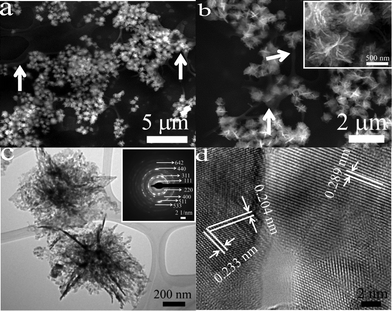
After calcination with PAN, the precursors were transformed into porous NiMn2O4/C tremella-like nanostructures, as shown in Fig. 4. It should be noted that after mixing with PAN, the hierarchical tremella-like structures are thermally stable, while the structures collapse after heat treatment without using PAN (Fig. S6†). Additionally, calcination treatment with PAN deposits non-uniform carbon films (marked with white arrows) on the tremella-like nanostructures as shown in Fig. 4a and b, which is beneficial for LIB applications. As the contrast of the carbon films differs considerably from that of the NiMn2O4 nanostructures, it is difficult to simultaneously show their images clearly. We have adjusted the contrast of Fig. 4a and b such that the carbon films can be more clearly seen in Fig. S7.† Meanwhile, the tremella-like nanostructure becomes porous and is composed of numerous nanoparticles as shown in the inset of Fig. 4b. EDS microanalysis of the NiMn2O4/C hierarchical tremella-like structures is shown in Fig. S8.† The nanostructures were found to contain only Ni, Mn, O and C, and the ratio of Ni and Mn is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, further confirming the formation of pure NiMn2O4. Also the C content was shown by FTIR and estimated to be 0.75 wt% based on the TGA analysis (Fig. S9†). In addition, energy dispersive X-ray spectroscopy mapping (Fig. S10†) shows the homogeneity of Ni, Mn and O in the tremella-like structures. The porous characteristic of the NiMn2O4/C hierarchical nanostructures was further examined via TEM observations (Fig. 4c). A Brunauer–Emment–Teller (BET) analysis of the porous NiMn2O4/C tremella-like structures gives a specific surface area of 38.9 m2 g−1 and a pore volume of 0.237 cm3 g−1 as shown in Fig. S11.† In addition, the SAED pattern (the inset in Fig. 4c) of the NiMn2O4 nanostructure shows its polycrystalline nature. An HRTEM image (Fig. 4d) shows lattice fringes with an interplanar spacing of 0.233, 0.204 and 0.289 nm corresponding to the (222), (400) and (220) planes of the spinel NiMn2O4 phase, respectively.
2, further confirming the formation of pure NiMn2O4. Also the C content was shown by FTIR and estimated to be 0.75 wt% based on the TGA analysis (Fig. S9†). In addition, energy dispersive X-ray spectroscopy mapping (Fig. S10†) shows the homogeneity of Ni, Mn and O in the tremella-like structures. The porous characteristic of the NiMn2O4/C hierarchical nanostructures was further examined via TEM observations (Fig. 4c). A Brunauer–Emment–Teller (BET) analysis of the porous NiMn2O4/C tremella-like structures gives a specific surface area of 38.9 m2 g−1 and a pore volume of 0.237 cm3 g−1 as shown in Fig. S11.† In addition, the SAED pattern (the inset in Fig. 4c) of the NiMn2O4 nanostructure shows its polycrystalline nature. An HRTEM image (Fig. 4d) shows lattice fringes with an interplanar spacing of 0.233, 0.204 and 0.289 nm corresponding to the (222), (400) and (220) planes of the spinel NiMn2O4 phase, respectively.
The electrochemical performance of the tremella-like NiMn2O4/C hierarchical nanostructures as anode materials for LIBs was first investigated by cyclic voltammetry (CV). Fig. 5a shows the first five CV curves of the NiMn2O4/C electrode at a scanning rate of 0.2 mV s−1 over the voltage range of 0.01–3.0 V. In the first cycle, the broad peak between 0.30 and 0.90 V can be attributed to the reduction of NiMn2O4 to Mn and Ni. In the anodic process, two broad oxidation peaks located at ∼1.25 V and ∼1.96 V are ascribed to the oxidation of Mn to Mn3+ and Ni to Ni2+.17,57 In the second cycle, the cathodic peaks at 1.02 and 0.53 V correspond to the reduction of Mn3+ to Mn2+ and Mn2+ or Ni2+ to metallic Mn or Ni, respectively. In the following cycles, the redox peaks move to lower potentials, while the anodic peaks show little changes. Apparently, the differences in the second cycle indicate a different electrochemical mechanism from the first anodic process. For the following cycles, the CV curves are almost overlapped, implying excellent electrochemical reversibility. Based on the above CV analysis, the electrochemical reactions for the NiMn2O4 electrode can be summarized as follows:17,57
| NiMn2O4 + 8Li+ + 8e− → Ni + 2Mn + 4Li2O | (1) |
| Ni + Li2O → NiO + 2Li+ + 2e− | (2) |
| Mn + Li2O → MnO + 2Li+ + 2e− | (3) |
| 2MnO + 1/3Li2O → 1/3Mn3O4 + 2/3Li+ + 2/3e− | (4) |
Fig. 5b shows typical discharge–charge curves of the LIBs with the NiMn2O4/C tremella-like anode at 1000 mA g−1 over a voltage range of 0.01–3.0 V. The initial discharge capacity is 1617 mA h g−1 with a corresponding Coulombic efficiency (CE) of 62.8%. The 37.2% capacity loss is mainly attributed to the formation of the solid electrolyte interphase (SEI) during the first discharge process.58 The discharge capacities in the 2nd and 150th cycles are 997 and 979 mA h g−1, respectively, which are both higher than the theoretical value (∼922 mA h g−1) of NiMn2O4. The high capacities could be attributed to synergistic effects, including the reversible formation/dissolution of polymeric gel-like films resulting from electrolyte degradation,59–63 which is often observed in transition metal oxides, and the interfacial storage of lithium ions46,47 and acetylene black.64
The porous NiMn2O4/C tremella-like structure electrode also exhibits remarkable rate performance (Fig. 5c). Upon cycling at current densities of 200, 500, 800, 1000, 2000 and 5000 mA g−1, the porous NiMn2O4/C nanostructure electrode shows discharge capacities of 1035, 948, 909, 900, 826 and 671 mA h g−1, respectively. The capacity can be retained at 1025 mA h g−1 as the current density reduces back to 200 mA g−1, indicating a good rate-cycling stability of this nanostructure. Long-term cycling performance of the porous NiMn2O4/C nanostructures was also measured at high current densities of 1000 mA g−1 and 4000 mA g−1 (Fig. 5d and e). At a current density of 1000 mA g−1, after the first cycle, the discharge capacity shows almost stable capacities of ∼900 mA h g−1 in the initial 30 cycles with the CE around ∼98%. Then the capacity decreases to a value of 680 mA h g−1 within 100 cycles. After that, it is interesting that the capacity increases gradually and can reach a high value of 2130 mA h g−1 after 300 cycles and then remains almost stable within 350 cycles. However, the pristine NiMn2O4 nanoparticles only show continuously decreasing capacities (Fig. S12†). So, a small amount of doped C can improve the electrochemical performance based on the fact of preserving the hierarchical structure and providing a continuous pathway for electron transport. To further evaluate the cycle stability of the NiMn2O4/C nanostructure electrode, we tested the long-term cycling performance at a much higher current density of 4000 mA g−1. As shown in Fig. 5e, the capacity also shows a similar trend to that at a current density of 1000 mA g−1. The electrode retains its capacity very well for 1500 cycles. The capacity in the 1500th cycle is still as high as 1773 mA h g−1, which is almost five times higher than the theoretical capacity of graphite.
The porous NiMn2O4/C tremella-like nanostructure in this work exhibits a superior electrochemical performance compared with other binary transition metal oxides for LIBs. The high capacity of this porous nanostructure should be attributed to the continuously reversible formation of a polymeric gel-like film originating from the progressive kinetic activation in the electrode. A similar phenomenon has also been reported by Maier et al.46 They pointed out that the interfacial storage is mainly from the reversible formation/dissolution of organic polymeric gel-like layers by electrolyte decomposition, which could deliver an extra capacity through a so-called pseudo-capacitive behavior. In each discharge process a fresh metal surface is generated due to the conversion characteristic of TMOs, and so additional electrolyte decomposition is needed to form SEI films. The electrolyte can thus penetrate into the inner part of the porous hierarchical nanostructures after several cycles. As a result, the inner part of the nanostructures is gradually involved in the conversion reactions and leads to the increased capacity. Nyquist plots of the electrode for the 1st and 150th cycles are shown in Fig. 6a. The charge-transfer resistance for the 150th cycle (∼3 Ω) was obviously smaller than that in the 1st cycle (∼7 Ω), which indicates the activation and improved kinetics upon cycling. In addition, through XPS analysis of the Mn peaks for the electrode material in the full charge state after 350 cycles at 1000 mA g−1 in Fig. 6b, we can observe the characteristic peak for Mn4+ at about 653.3 eV and 642.2 eV, while these peaks cannot be observed in the as-prepared NiMn2O4/C. This suggests that some Mn2+ or Mn3+ ions can be re-oxidized to a higher oxidation state (e.g. Mn4+) upon cycling, which also leads to an increased capacity according to Huang's report.50
To understand the relationship between the improved performances and structure, we also studied the morphological changes in the porous NiMn2O4/C tremella-like nanostructure electrode upon cycling (Fig. 6c and d). After 350 discharge/charge cycles at 1000 mA g−1, the tremella-like nanostructures changed into porous clusters (Fig. 6c) composed of many nanosized particles, promoting the surface Li storage capacities, and thus led to a capacity increase during the discharge process.46 On the other hand, although the tremella-like morphology changed upon cycling, the porous characteristic of the formed clusters still provides sufficient contact with the electrolyte and three-dimensional channels for Li+ diffusion (Fig. 6d), which should be attributed to the long life capacity stability of this porous NiMn2O4/C tremella-like hierarchical nanostructure.
4. Conclusions
Porous tremella-like NiMn2O4/C hierarchical nanostructures can be facilely obtained through thermal annealing treatment of the synthesized Ni1/3Mn2/3CO3 precursor with a carbon source of PAN. As anode materials, the porous NiMn2O4/C nanostructure electrode shows a superior capacity of 2130 mA h g−1 within 350 cycles at a current density of 1000 mA g−1. Even at a high current of 4000 mA g−1, the capacity can reach as high as 1773 mA h g−1 after 1500 cycles, which is almost five times higher than the theoretical capacity of graphite. The excellent electrochemical performance can be attributed to the porous hierarchical structures of NiMn2O4/C with a specific surface area of 38.9 m2 g−1, which is in favor of the interfacial lithium storage state upon cycling. Further oxidation of transition metals to higher oxidization states can also contribute to the increased capacity. It is believed that the high rate capacity and cycling stability of this porous NiMn2O4/C nanostructure will make it a promising anode candidate for high performance LIB applications.Acknowledgements
This project has been financially supported by the National Natural Science Foundation of China (no. 51272217 and 51302238), Collaboration Project of City University of Hong Kong and Shenzhen Huawei (YB2012090343), and Guangdong Innovative and Entrepreneurial Research Team Program (no. 2013C090).Notes and references
- S. J. Guo and E. K. Wang, Nano Today, 2011, 6, 240 CrossRef CAS PubMed.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- C. Z. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488 CrossRef CAS PubMed.
- J. J. Wu, W. P. Liao and M. Yoshimura, Nano Energy, 2013, 2, 1354 CrossRef CAS PubMed.
- Y. Li, Z. Y. Fu and B. L. Su, Adv. Funct. Mater., 2012, 22, 4634 CrossRef CAS.
- C. M. A. Parlett, K. Wilson and A. F. Lee, Chem. Soc. Rev., 2013, 42, 3876 RSC.
- S. Jin, H. Deng, D. Long, X. Liu, L. Zhan, X. Liang, W. Qiao and L. Ling, J. Power Sources, 2011, 196, 3887 CrossRef CAS PubMed.
- D. W. Liu, B. B. Garcia, Q. F. Zhang, Q. Guo, Y. H. Zhang, S. Sepehri and G. Z. Cao, Adv. Funct. Mater., 2009, 19, 1015 CrossRef CAS.
- T. Y. Wei, C. H. Chen, H. C. Chien, S. Y. Lu and C. C. Hu, Adv. Mater., 2010, 22, 347 CrossRef CAS PubMed.
- Z. Yang, Y. Xia and R. Mokaya, J. Am. Chem. Soc., 2007, 129, 1673 CrossRef CAS PubMed.
- L. Xu, S. Sithambaram, Y. Zhang, C. H. Chen, L. Jin, R. Joesten and S. L. Sui, Chem. Mater., 2009, 21, 1253 CrossRef CAS.
- M. Prabu, K. Ketpang and S. Shanmugam, Nanoscale, 2014, 6, 3173 RSC.
- Y. F. Zhao, M. Wei, J. Lu, Z. L. Wang and X. Duan, ACS Nano, 2009, 3, 4009 CrossRef CAS PubMed.
- X. Y. Yu, T. Luo, Y. Jia, R. X. Xu, C. Gao, Y. X. Zhang, J. H. Liu and X. J. Huang, Nanoscale, 2012, 4, 3466 RSC.
- L. H. Ai, H. T. Yue and J. Jiang, Nanoscale, 2012, 4, 5401 RSC.
- L. L. Wang, W. Cheng, H. X. Gong, C. H. Wang, D. K. Wang, K. B. Tang and Y. T. Qian, J. Mater. Chem., 2012, 22, 11297 RSC.
- J. F. Li, S. L. Xiong, X. W. Li and Y. T. Qian, Nanoscale, 2013, 5, 2045 RSC.
- S. L. Xiong, J. S. Chen, X. W. Lou and H. C. Zeng, Adv. Funct. Mater., 2012, 22, 861 CrossRef CAS.
- W. P. Kang and Q. Shen, J. Power Sources, 2013, 238, 203 CrossRef CAS PubMed.
- M. Tadic, S. M. Savic, Z. Jaglicic, K. Vojisavljevic, A. Radojkovic, S. Prsic and D. Nikolic, J. Alloys Compd., 2014, 588, 465 CrossRef CAS PubMed.
- Menaka, N. Garg, S. Kumar, D. Kumar, K. V. Ramanujachary, S. E. Loflandc and A. K. Ganguli, J. Mater. Chem., 2012, 22, 18447 RSC.
- S. Fritsch, J. Sarrias, M. Brieu, J. J. Couderc, J. L. Baudour, E. Snoeck and A. Rousset, Solid State Ionics, 1998, 109, 229 CrossRef CAS.
- D. Mehandjiev, E. Zhecheva, G. Ivanov and R. Ioncheva, Appl. Catal., A, 1998, 167, 277 CrossRef CAS.
- D. L. Fang, Z. B. Wang, P. H. Yang, W. Liu and C. S. Chen, J. Am. Ceram. Soc., 2006, 89, 230 CrossRef CAS PubMed.
- H. Pang, J. W. Deng, S. M. Wang, S. J. Li, J. M. Du, J. Chen and J. S. Zhang, RSC Adv., 2012, 2, 5930 RSC.
- M. Zhang, S. H. Guo, L. Zheng, G. N. Zhang, Z. P. Hao, L. P. Kang and Z. H. Liu, Electrochim. Acta, 2013, 87, 546 CrossRef CAS PubMed.
- W. H. Cloud, Phys. Rev., 1958, 111, 1043 CrossRef.
- A. Ashcroft, I. Terry and R. Gover, J. Eur. Ceram. Soc., 2006, 26, 901 CrossRef PubMed.
- J. M. A. Almeida, C. T. Meneses, A. S. de Menezes, R. F. Jardim and J. M. Sasaki, J. Magn. Magn. Mater., 2008, 320, 304 CrossRef PubMed.
- G. Q. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609 CrossRef CAS PubMed.
- L. Zhou, D. Y. Zhao and X. W. Lou, Adv. Mater., 2012, 24, 745 CrossRef CAS PubMed.
- L. Zhou, H. B. Wu, T. Zhu and X. W. Lou, J. Mater. Chem., 2012, 22, 827 RSC.
- G. Fortunato, H. R. Oswald and A. Reller, J. Mater. Chem., 2001, 11, 905 RSC.
- F. M. Courtel, Y. Abu-Lebdeh and I. J. Davidson, Electrochim. Acta, 2012, 71, 123 CrossRef CAS PubMed.
- F. M. Courtel, H. Duncan, Y. Abu-Lebdeh and I. J. Davidson, J. Mater. Chem., 2011, 21, 10206 RSC.
- Y. G. Wang, H. Q. Li, P. He, E. Hosono and H. S. Zhou, Nanoscale, 2010, 2, 1294 RSC.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS PubMed.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364 CrossRef CAS PubMed.
- W. M. Zhang, X. L. Wu, J. S. Hu, Y. G. Guo and L. J. Wan, Adv. Funct. Mater., 2008, 18, 3941 CrossRef CAS.
- Z. S. Wu, W. C. Ren, L. Wen, L. B. Gao, J. P. Zhao, Z. P. Chen, G. M. Zhou, F. Li and H. M. Cheng, ACS Nano, 2010, 4, 3187 CrossRef CAS PubMed.
- J. Y. Wang, N. L. Yang, H. J. Tang, Z. H. Dong, Q. Jin, M. Yang, D. Kisailus, H. J. Zhao, Z. Y. Tang and D. Wang, Angew. Chem., Int. Ed., 2013, 52, 6417 CrossRef CAS PubMed.
- R. Alcántara, M. Jaraba, P. Lavela and J. L. Tirado, Chem. Mater., 2002, 14, 2847 CrossRef.
- S. H. Choi and Y. C. Kang, ChemSusChem, 2013, 6, 2111 CrossRef CAS PubMed.
- L. Yu, L. Zhang, H. B. Wu, G. Q. Zhang and X. W. Lou, Energy Environ. Sci., 2013, 6, 2664 CAS.
- J. Maier, Nat. Mater., 2005, 4, 805 CrossRef CAS.
- P. Balaya, H. Li, L. Kienle and J. Maier, Adv. Funct. Mater., 2003, 13, 621 CrossRef CAS.
- V. Augustyn, P. Simonbc and B. Dunn, Energy Environ. Sci., 2014, 7, 1597 CAS.
- J. F. Li, J. Z. Wang, X. Liang, Z. J. Zhang, H. K. Liu, Y. T. Qian and S. L. Xiong, ACS Appl. Mater. Interfaces, 2014, 6, 24 CAS.
- Y. M. Sun, X. L. Hu, W. Luo, F. F. Xia and Y. H. Huang, Adv. Funct. Mater., 2013, 23, 2436 CrossRef CAS.
- S. Luo, H. C. Wu, Y. Wu, K. L. Jiang, J. P. Wang and S. S. Fan, J. Power Sources, 2014, 249, 463 CrossRef CAS PubMed.
- W. P. Kang, C. H. Zhao, R. Liu, F. F. Xu and Q. Shen, CrystEngComm, 2012, 14, 2245 RSC.
- Y. L. Wang, X. C. Jiang and Y. N. Xia, J. Am. Chem. Soc., 2003, 125, 16176 CrossRef CAS PubMed.
- Y. C. Qiu, S. H. Yang, H. Deng, L. M. Jin and W. S. Li, J. Mater. Chem., 2010, 20, 4439 RSC.
- J. F. Marco, J. R. Gancedo, M. Gracia, J. L. Gautier, E. I. Ríos, H. M. Palmer, C. Greaves and F. J. Berry, J. Mater. Chem., 2001, 11, 3087 RSC.
- B. Cui, H. Lin, Y. Z. Liu, J. B. Li, P. Sun, X. C. Zhao and C. J. Liu, J. Phys. Chem. C, 2009, 113, 14083 CAS.
- L. L. Li, Y. Y. Cheah, Y. Ko, P. F. Teh, G. Wee, C. L. Wong, S. J. Peng and M. Srinivasan, J. Mater. Chem. A, 2013, 1, 10935 CAS.
- W. P. Kang, F. L. Liu, Y. L. Su, D. J. Wang and Q. Shen, CrystEngComm, 2011, 13, 4174 RSC.
- R. Demir-Cakan, Y. S. Hu, M. Antonietti, J. Maier and M. M. Titirici, Chem. Mater., 2008, 20, 1227 CrossRef CAS.
- H. Wang, L. F. Cui, Y. Wang, H. S. Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Gai, J. Am. Chem. Soc., 2010, 132, 13978 CrossRef CAS PubMed.
- B. Z. Jang, C. Liu, D. Neff, Z. Yu, M. C. Wang, W. Xiong and A. Zhamu, Nano Lett., 2011, 11, 3785 CrossRef CAS PubMed.
- S. W. Lee, N. Yabuuchi, B. M. Gallant, S. Chem, B. S. Kim, P. T. Hammond and Y. Shao-Horn, Nat. Naonotechnol., 2010, 5, 531 CrossRef CAS PubMed.
- H. C. Liu and S. K. Yen, J. Power Sources, 2007, 166, 478 CrossRef CAS PubMed.
- Y. Deng, Q. Zhang, S. Tang, L. Zhang, S. Deng, Z. Shi and G. Chen, Chem. Commun., 2011, 47, 6828 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nr04031g |
| This journal is © The Royal Society of Chemistry 2015 |