One-pot solvothermal synthesis of graphene wrapped rice-like ferrous carbonate nanoparticles as anode materials for high energy lithium-ion batteries
Fan
Zhang
a,
Ruihan
Zhang
a,
Jinkui
Feng
*a,
Lijie
Ci
a,
Shenglin
Xiong
b,
Jian
Yang
b,
Yitai
Qian
*b and
Lifei
Li
*b
aKey Laboratory for Liquid-Solid Structural Evolution & Processing of Materials (Ministry of Education), School of Materials Science and Engineering, Shandong University, Jinan 250061, China
bSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, PR China. E-mail: jinkuifeng@sdu.edu.cn
First published on 6th November 2014
Abstract
Well dispersed rice-like FeCO3 nanoparticles were produced and combined with reduced graphene oxide (RGO) via a one-pot solvothermal route. SEM characterization shows that rice-like FeCO3 nanoparticles are homogeneously anchored on the surface of the graphene nanosheets; the addition of RGO is helpful to form a uniform morphology and reduce the particle size of FeCO3 to nano-grade. As anode materials for lithium-ion batteries, the FeCO3/RGO nanocomposites exhibit significantly improved lithium storage properties with a large reversible capacity of 1345 mA h g−1 for the first cycle and a capacity retention of 1224 mA h g−1 after 50 cycles with a good rate capability compared with pure FeCO3 particles. The superior electrochemical performance of the FeCO3/RGO nanocomposite electrode compared to the pure FeCO3 electrode can be attributed to the well dispersed RGO which enhances the electronic conductivity and accommodates the volume change during the conversion reactions. Our study shows that the FeCO3/RGO nanocomposite could be a suitable candidate for high capacity lithium-ion batteries.
1. Introduction
Lithium-ion batteries (LIBs) have become one of the most promising energy storage devices because of their high energy density, light weight and low cost.1–4 Due to the low theoretical capacity and unsatisfactory high-rate performances of commercialized graphite anodes, numerous efforts have been made to develop alternative high-performance anode materials for the next generation LIBs. For example, transition-metal based materials have been proven to be potential candidates as anode materials for lithium ion batteries, including various transition-metal oxides (such as Co3O4,5,6 Fe3O47,8 and MnO29) and their oxysalts (such as transition-metal germanates,10–12 oxalates13,14 and carbonates15–26), because of their high theoretical specific capacity, natural abundance and intrinsic safety.As a family of important functional materials, transition metal carbonates have attracted much attention as they have potential applications in catalyst support, surface engineering, gas adsorption and separation and so on.27–29 Beyond that, transition metal carbonates have also been verified to be promising anode materials for lithium ion batteries. Aragón et al. first carried out the analysis of the electrochemical performance of MnCO3. Its initial reversible capacity was 670 mA h g−1 when cycled at the current rate of 0.25 C in the voltage range 0–3.0 V.15 Later, Ding et al. further verified that CoCO3, ZnCO3, CuCO3 and their composites could also deliver a reversible capacity as high as 800 mA h g−1, enabling transition metal carbonates to be novel high capacity anode materials.17–23 It is worth noting that the observed reversible capacities of transition metal carbonates are always higher than their theoretical capacities calculated from electrochemical reactions and the extra capacity could be ascribed to the capacitive contribution of Faradic and non-Faradic reasons.15–26 For example, Su discovered that CoCO3 microspheres exhibited a reversible capacity of 930 mA h g−1 (40 cycles, at 1C), which was higher than its theoretical value (450 mA h g−1); he verified that not only Co2+ but also CO32− ions are involved in the electron transfer, C4+ in CO32− is reduced to C0 or other low-valence C under the electrochemical catalysis of newly generated Co nanoparticles.23 However, capacity-fading was observed for these transition metal carbonates. Taking MnCO3 as an example, a capacity of only 450 mA h g−1 was noted after 25 cycles, which is due to the huge volume change during the conversion process and the poor electrical conductivity of carbonates.15
As we know, using conductive active materials or adding conductive substrates such as graphene or metals could optimize the electrochemical performances of anode materials.30–35 Reduced graphene oxide (RGO) is well known due to its special 2D structure, superior electronic conductivity and large specific surface area. By combining with RGO, the electrochemical performance of anode materials could be greatly enhanced.33–36 As reported by Xia, the CoFe2O4/RGO nanocomposite electrode can deliver a high reversible specific capacity of up to 1082 mA h g−1 as well as better cycling stability and rate capability than that of the pure CoFe2O4 nanoparticles. The RGO not only helps in relieving the volume change of the active materials but also offers an enhanced electrical contact.35
Among transition metal carbonates, FeCO3 has many obvious advantages, for example low cost, abundant resources, green character and high theoretical capacity; moreover, it can be used as a precursor to prepare Fe3O4. Assuming that not only Fe2+ will reduce to metallic Fe0+, but also C4+ in CO32− can be reduced to C0 in the process of electrochemical reaction, pure FeCO3, which has a very high theoretical capacity about 1620 mA h g−1, can be a suitable anode material for high capacity lithium-ion batteries.22–24 Based on these concepts, we have developed a facile one-pot solvothermal method to synthesize the FeCO3/RGO nanocomposite, which consists of rice-like FeCO3 nanoparticles and reduced graphene sheets. According to our observations, RGO is helpful for the FeCO3 crystal to attain a uniform morphology and reduce the particle size to nano-grade. When used as the anode material for LIBs, the FeCO3/RGO nanocomposite exhibits a large reversible capacity of 1345 mA h g−1 for the first cycle and a capacity retention of 1224 mA h g−1 after 50 cycles with a good rate capability, which are all better than those of the pure FeCO3 electrode.
2. Experimental
2.1. Synthesis of graphene oxide, rice-like FeCO3 nanoparticles and FeCO3/RGO nanocomposites
All the concerned reagents were of analytic grade and used without further purification. Graphene oxide (GO) was synthesized from natural flake graphite power through a modified Hummers method. Rice-like FeCO3 nanoparticles: in a typical procedure, 1.08 g FeCl3·6H2O was completely dissolved in 50 mL distilled water and stirred for 30 min. Next, 24 mL 0.5 M Na2CO3 aqueous solution was added dropwise. Then, 1.00 g ascorbic acid was added to the above solution and stirred for 30 min. Finally, the mixture was transferred into a stainless steel autoclave with a Teflon liner of 100 mL capacity and heated in an oven at 160 °C for 12 h, followed by cooling to room temperature. FeCO3/RGO nanocomposites: 40 mg graphene oxide was ultrasonicated in 40 mL deionized water to form suspension A. 1.08 g FeCl3·6H2O was completely dissolved in 40 mL ethylene glycol (EG)–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) under continuous magnetic stirring for 30 min to form solution B. Then suspension A and solution B were mixed together uniformly. Next, 1.27 g Na2CO3 was added under stirring. Then, 1.00 g ascorbic acid was added to the above solution and stirred for 30 min. Finally, the mixture was transferred into a 100 mL Teflon-sealed autoclave and heated in an oven at 160 °C for 12 h, followed by cooling to room temperature. The obtained precipitates were separated by filtration, and washed with distilled water and ethanol several times and dried at 50 °C for 6 h. The preparation procedure of FeCO3/RGO nanocomposites is illustrated in Fig. 1.
1 v/v) under continuous magnetic stirring for 30 min to form solution B. Then suspension A and solution B were mixed together uniformly. Next, 1.27 g Na2CO3 was added under stirring. Then, 1.00 g ascorbic acid was added to the above solution and stirred for 30 min. Finally, the mixture was transferred into a 100 mL Teflon-sealed autoclave and heated in an oven at 160 °C for 12 h, followed by cooling to room temperature. The obtained precipitates were separated by filtration, and washed with distilled water and ethanol several times and dried at 50 °C for 6 h. The preparation procedure of FeCO3/RGO nanocomposites is illustrated in Fig. 1.
2.2. Sample characterization
The crystal structures and chemical compositions of samples were characterized using X-ray diffraction (XRD, Rigaku Dmaxrc diffractometer, V = 50 kV, I = 100 mA) at a scanning rate of 5 degree min−1 and Raman spectroscopy. The morphologies of the products were examined by SU-70 thermal field emission scanning electron microscopy (FESEM) and JEM-2100 high resolution transmission electron microscopy (HRTEM). Thermogravimetric analysis (TGA) was performed on a Mettler-Toledo TGA/SDTA851e Thermo Analyzer from room temperature to 800 °C at a rate of 5 °C min−1. Raman spectra were recorded on a Horiba Jobin-YVON co-focal laser Raman system with a He–Ne 632 nm laser as the excitation source.2.3. Electrochemical test
The electrochemical performance was measured in 2016 coin-type cells. To prepare the working electrode, 70 wt% active material (FeCO3 nanoparticles and FeCO3/RGO nanocomposites), 20 wt% carbon black and 10 wt% carboxy methyl cellulose sodium (CMC) dissolved uniformly in distilled water were mixed to form a slurry. The resulting paste was cast on a Cu foil substrate and dried in a vacuum oven at 90 °C for 24 h to form the electrodes. Li foil was used as the counter electrode, and Celgard 2400 as the separator. The electrolyte used was 1 M LiPF6 dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ethylene carbonate, diethyl carbonate and dimethyl carbonate with 5% fluoro-ethyl carbonate (FEC). The cells were assembled in an atmosphere of high-purity argon in a glove box. Galvanostatic discharge–charge measurements of the cells were carried out in a voltage range of 0.01–3 V at various rates. Cyclic voltammetry (CV) tests were performed at a scanning rate of 0.1 mV s−1 between 0.01 and 3.0 V (vs. Li/Li+).
1 mixture of ethylene carbonate, diethyl carbonate and dimethyl carbonate with 5% fluoro-ethyl carbonate (FEC). The cells were assembled in an atmosphere of high-purity argon in a glove box. Galvanostatic discharge–charge measurements of the cells were carried out in a voltage range of 0.01–3 V at various rates. Cyclic voltammetry (CV) tests were performed at a scanning rate of 0.1 mV s−1 between 0.01 and 3.0 V (vs. Li/Li+).
3. Results and discussion
Fig. 2(a) shows powder XRD patterns of pure FeCO3 and FeCO3/RGO nanocomposite samples, respectively. All the diffraction peaks can be indexed to a rhombohedral FeCO3 crystal (JCPDS no. 12-0531) with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) C space group. FeCO3/RGO nanocomposites show almost the same diffraction as FeCO3; since the diffraction from RGO is too weak to be observed, due to the nanocrystalline nature of the particles, the peaks show some broadening. There are no other miscellaneous peaks confirming that the FeCO3 in FeCO3/RGO nanocomposites is also in the pure phase.37Fig. 2(b) shows the Raman spectra of the pure FeCO3 microparticles, GO and FeCO3/RGO nanocomposites. Pure FeCO3 shows no obvious feature peaks, while the Raman spectra of the GO and FeCO3/RGO nanocomposites display G bands corresponding to the first-order scattering of the E2g phonon of sp2 C atoms and D bands which are ascribed to edge planes and disordered structures.38 Compared with the Raman spectrum of the GO, it is obvious that the Raman peaks of G- and D-bands in the Raman spectra of the FeCO3/RGO nanocomposites shift to lower frequencies. The D-band shifted from 1365 to 1350 cm−1 while the G-band shifted from 1608 to 1592 cm−1, indicating that GO has been reduced to graphene.39,40 It has been reported that the intensity ratio of the D-band to the G-band (ID/IG) increases when GO is reduced to graphene.38 For our samples, the ratio of ID/IG increased from 1.22 of GO to 1.42 of FeCO3/RGO nanocomposites. The reason for this phenomenon might be that most of the oxygen-containing groups are removed during the reduction process, and the conjugated G network is re-established. However, the size of the re-established G network is smaller than the original one, which results in an increase in the ID/IG ratio.45Fig. 2(c) shows the TGA curve of the FeCO3/RGO nanocomposites. The weight loss between 200 and 600 °C can be attributed to the decomposition of FeCO3 to Fe2O3 and the oxidation of carbon, subtracting the contribution from the theoretical weight loss of FeCO3; the graphene weight ratio in the composites was estimated to be 16.1%.
C space group. FeCO3/RGO nanocomposites show almost the same diffraction as FeCO3; since the diffraction from RGO is too weak to be observed, due to the nanocrystalline nature of the particles, the peaks show some broadening. There are no other miscellaneous peaks confirming that the FeCO3 in FeCO3/RGO nanocomposites is also in the pure phase.37Fig. 2(b) shows the Raman spectra of the pure FeCO3 microparticles, GO and FeCO3/RGO nanocomposites. Pure FeCO3 shows no obvious feature peaks, while the Raman spectra of the GO and FeCO3/RGO nanocomposites display G bands corresponding to the first-order scattering of the E2g phonon of sp2 C atoms and D bands which are ascribed to edge planes and disordered structures.38 Compared with the Raman spectrum of the GO, it is obvious that the Raman peaks of G- and D-bands in the Raman spectra of the FeCO3/RGO nanocomposites shift to lower frequencies. The D-band shifted from 1365 to 1350 cm−1 while the G-band shifted from 1608 to 1592 cm−1, indicating that GO has been reduced to graphene.39,40 It has been reported that the intensity ratio of the D-band to the G-band (ID/IG) increases when GO is reduced to graphene.38 For our samples, the ratio of ID/IG increased from 1.22 of GO to 1.42 of FeCO3/RGO nanocomposites. The reason for this phenomenon might be that most of the oxygen-containing groups are removed during the reduction process, and the conjugated G network is re-established. However, the size of the re-established G network is smaller than the original one, which results in an increase in the ID/IG ratio.45Fig. 2(c) shows the TGA curve of the FeCO3/RGO nanocomposites. The weight loss between 200 and 600 °C can be attributed to the decomposition of FeCO3 to Fe2O3 and the oxidation of carbon, subtracting the contribution from the theoretical weight loss of FeCO3; the graphene weight ratio in the composites was estimated to be 16.1%.
The morphologies of products were characterized by FE-SEM and HRTEM as shown in Fig. 3. It can be seen from Fig. 3(a) that FeCO3 microparticles with irregular shape and severe aggregation were produced after the solvothermal treatment, the size of FeCO3 microparticles is in the range of 150–250 nm. When RGO was introduced in FeCO3 (Fig. 3(b)), most of the rice-like FeCO3 nanoparticles with a typical diameter of 50 nm and the length in the range of 80–120 nm were uniformly dispersed on the graphene nanosheets with almost no aggregation. The high magnification FE-SEM images (Fig. 3(c) and 3(d)) clearly show the details of the morphology of FeCO3/RGO nanocomposites. The almost transparent two-dimensional graphene nanosheets act as supporting substrates for homogeneously anchoring FeCO3 nanoparticles, building a FeCO3–RGO heteroarchitecture. It is speculated that the addition of RGO could lead to uniform distribution of FeCO3 nanoparticles on the graphene nanosheets and suppress the aggregation of FeCO3 nanoparticles.35,41Fig. 3(e) shows the HR-TEM image of FeCO3/RGO nanocomposites and the corresponding SAED pattern, obtained from individual nanoparticles, indicating that these rice-like FeCO3 nanoparticles are monocrystalline. As obviously observed in the representative HRTEM image (Fig. 3(f)), the (006) lattice spacing (0.254 nm) verified the rhombohedral FeCO3 crystal texture.
The electrochemical performances of pure FeCO3 microparticles and FeCO3/RGO nanocomposites were characterized by static-current charge–discharge. The voltage profiles of the 1st, 2th, 10th, 20th and 50th cycles for the pure FeCO3 microparticles and FeCO3/RGO nanocomposite electrodes cycled between 0.01 and 3 V at a current density of 100 mA g−1 are shown in Fig. 4(a) and 4(b). For the pure FeCO3 electrode (Fig. 4(a)), during the initial cycle within a range of 0.01–3 V, a high specific discharge and charge capacity of 1820 mA h g−1 and 1310 mA h g−1 can be achieved, respectively. The irreversible capacity results from the well-known formation of a solid electrolyte interface (SEI) film and other irreversible reactions. The main plateau of SEI is above 0.5 V. The declining plateau from 0.45 V in the first discharge curve is attributed to the conversion reduction of Fe2+ to metallic Fe0+ accompanied by the reduction of C4+ to C0+ then to Li2C2.23–25 During the following de-lithiation process the cell voltage shows a sloppy signature centered at about 1.7 V vs. Li. The above mentioned reduction plateau vanished upon lithiation, illustrating a different reaction mechanism. The reversible capacity remains only 680 mA h g−1 after 50 cycles. As a comparison, for FeCO3/RGO, there was no such plateau formed at 0.45 V but a slope was formed at about 1.3 V, which is ascribed to the lithiation process of RGO accompanied by the formation of a SEI film.7 The plateau of FeCO3/RGO is not obvious compared with pure FeCO3. The reason for this phenomenon is the high electronic conductivity of FeCO3/RGO nanocomposites, which facilitates the phase transformation during the lithiation/de-lithiation progress.7–9 The capacity retention of FeCO3/RGO (Fig. 4(b)) is as high as 1210 mA h g−1 after 50 cycles. The high reversible capacity is from the conversion mechanism of Fe2+ to Fe and C4+ to Li2C2.23–25 From the cycling result we can see that there is an increase in the capacity at about 30 cycles, which is well recognized to be an electrode activation process.7,8
Fig. 4(e) compares the cycle and rate performances of the pure FeCO3 microparticles and FeCO3/RGO nanocomposites. For cycle performances, both electrodes are cycled between 0.01 and 3 V at different current densities for 100 cycles. It can be seen that the FeCO3/RGO nanocomposite electrode exhibits much better cycling stability than that of the pure FeCO3 electrode. The capacity remains 1224 mA h g−1 for 50 cycles and 1250 mA h g−1 for 100 cycles, which correspond to 91.0% and 93.2% capacity retention; the obtained reversible capacity of the FeCO3/RGO nanocomposite electrode is much higher than the theoretical capacity of commercially used graphite anodes (372 mA h g−1). As shown in Fig. 4(c) and 4(d), the pure graphene electrode can deliver a reversible capacity of about 415 mA h g−1 for the first cycle and 352 mA h g−1 after 100 cycles. Therefore, the graphene content in the nanocomposite should be controlled in a certain range, otherwise a considerably high graphene content could sacrifice the high specific reversible capacity.35 For our experiment, subtracting the contribution from the RGO in FeCO3/RGO nanocomposites, a reversible capacity about 1479 mA h g−1 can be attributed to the 84.9 wt% FeCO3 after 100 cycles, which is basically close to the theoretical capacities of pure FeCO3 (1620 mA h g−1). It is worth noting that the discharge capacity of the FeCO3/RGO nanocomposite electrode appeared to increase slightly after 25 cycles; such an interesting phenomenon is commonly observed for many metal oxides and their oxysalts, which can be attributed to the activation process for the electrochemical reaction of lithium, along with the formation of a polymeric/gel-like film.42–44 In comparison, the capacity decay for the pure FeCO3 electrode is much serious, only 70.8% (765 mA h g−1) and 62.7% (698 mA h g−1) capacity remained. Such a significant capacity fading for the pure FeCO3 electrode may be due to its poor electronic conductivity and it tends to collapse along with repeated electrochemical reaction processes. In addition, the obtained pure FeCO3 were microparticles of large sizes, which seriously restricted their potential for lithium storage. In addition to the better capacity retention, the FeCO3/RGO nanocomposite electrode also exhibits superior rate capacities as shown in Fig. 4(c). With an increase in current density, the FeCO3/RGO electrode can deliver a reversible capacity of 1224, 1132, 1045 and 974 mA h g−1 at a current density of 0.1, 0.5, 1.0 and 1.5 A g−1, respectively. A reversible capacity of 1250 mA h g−1 can be achieved after the rate returns to 0.1 A g−1. In contrast, the capacities of pure FeCO3 microparticles prepared under the same conditions were only 765, 610, 423 and 314 mA h g−1, respectively. A reversible capacity of 698 mA h g−1 can be achieved after the rate returns to 0.1 A g−1. Hence, the result demonstrates that the structure of the nanocomposite is very stable, and the Li+ ion insertion/extraction process is quite reversible even at high current rates. It is believed that the addition of RGO could not only suppress the aggregation of FeCO3 nanoparticles but also prevent the restacking of graphene nanosheets, resulting in a large electrode/electrolyte interface area, which facilitates fast lithium ion and electron transport, and RGO can serve as an inactive confining buffer to accommodate the volume change during electric cycling. All of these lead to a better electrochemical performance of the FeCO3/RGO nanocomposite electrode.
In order to gain better understanding of why FeCO3/RGO nanocomposites exhibit such a superior electrochemical performance compared to the pure FeCO3 electrode, EIS measurements were performed as shown in Fig. 4(f). The high frequency semicircle corresponds to the charge-transfer resistance Rct and CPE of the electrode–electrolyte interface, and the inclined line at about 45–50° angle to the real axis corresponds to the lithium-diffusion process. It shows that the Rct of the FeCO3/RGO electrode is 210 Ω, which is significantly lower than that of the pure FeCO3 electrode (Rct = 430 Ω). It is believed that the high electrical conductivity of the graphene nanosheets could be maintained in the nanocomposite sample and holds the FeCO3 nanoparticles tightly in the pores. This action prevents the FeCO3 nanoparticles from aggregating and thereby enlarges the contact area between the electrode and the electrolyte, resulting in a significant improvement in the kinetic performance of electrochemical lithium insertion/extraction. Therefore, the FeCO3/RGO nanocomposite electrode exhibits a considerably enhanced capacity with excellent cycle stability and rate capability.
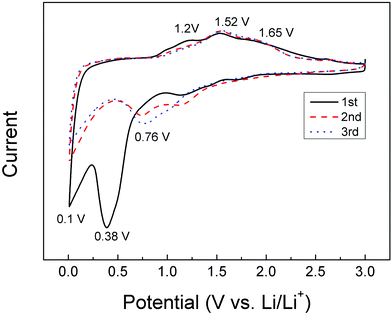
Cyclic voltammograms were further obtained to investigate the electrochemical behaviors of FeCO3/RGO nanocomposites (Fig. 5). The initial cathodic scan reveals two main peaks from 1.3 V, 0.9 V and 0.3 V, in agreement with the initial discharge curve. The first small peak is attributed to Fe2+ to Fe0 and the formation of a SEI film, which coincides with many iron oxides. The peak at 0.1 V is attributed to the combination of complete reduction of Fe2+ to Fe0 and the CO32− decomposition.22–25 For the anodic scan, the hump-like peaks at 1.2 and 1.52 V, and the succeeding broad anodic peaks centered at 1.65 V, are due to the further oxidation of metallic Fe0 to Fe3+, accompanied by the re-oxidation of low valence C. The reduction peaks at 0.38 V are replaced by two broad split peaks shifting to 0.92 V with a decreased intensity while the peak at 0.1 V remains in the second cycle, indicating partly irreversible processes in the first cycle. Interestingly, the reduction peaks are replaced by a broad split peak shifting to 0.76 V with a decreased intensity in the third cycle, which is ascribed to the amorphous nature/crystal structure destruction of the material, reported previously for some metal oxysalts and metal oxides.23–25 Moreover, the subsequent CV curves overlap well, revealing good reversibility of Li storage performances.
The mechanical stability of the FeCO3/RGO composite was further investigated by ex situ SEM imaging for electrodes that were removed from a cell after 100 charge/discharge cycles. Cracks and aggregation on the surface of the pure FeCO3 electrode are observed in Fig. 6(a) and (b), which cause the disconnection of the active materials with the current collector and result in capacity fading. However, no disassembly or cracking of the FeCO3/RGO composite was found (Fig. 6(c) and (d)), which shows that the FeCO3 was still uniformly anchored onto the electrode after cycling.
 | ||
| Fig. 6 Ex situ FE-SEM images of cycled electrodes (a and b), pure FeCO3 microparticles and (c and d) FeCO3/RGO nanocomposites. | ||
The greatly enhanced electrochemical performance of the FeCO3/RGO electrode is comparable to that of a bare FeCO3 electrode may be due to the following factors: (1) the graphene coating on the FeCO3 provides a robust elastic buffer to accommodate a large volume change thus maintaining the integrity of individual FeCO3 particles. (2) The RGO network provides a block effect for the aggregation of FeCO3 particles, yielding a higher mechanical strength to better withstand the stress buildup in the electrode; (3) the RGO greatly improved the electronic conductivity and catalysed the conversion reaction of FeCO3, which was proved by Wang's group.34
4. Conclusions
A facile solvothermal method has been developed to synthesise FeCO3 microparticles and FeCO3/RGO nanocomposites. When evaluated as anode materials for lithium-ion batteries, the nanocomposites exhibit high reversible capacity, good cycling performance and good rate capability. The superior electrochemical performance of the nanocomposite electrode is due to the high electronic conductivity and the high specific area of RGO, which buffer a large volume change and enable fast ion and electron transport. These FeCO3/RGO nanocomposites could be used as promising electrode materials for lithium-ion batteries.References
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- W. H. Li, Z. Z. Yang, J. X. Cheng, X. W. Zhong, L. Gu and Y. Yu, Nanoscale, 2014, 6, 4532–4537 RSC.
- M. G. Kim and J. Cho, Adv. Funct. Mater., 2009, 19, 1497–1514 CrossRef CAS.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–372 CrossRef CAS PubMed.
- J. Liu, H. Xia, L. Lu and D. F. Xue, J. Mater. Chem., 2010, 20, 1506–1510 RSC.
- G. L. Xu, J. T. Li, L. Huang, W. Lin and S. G. Sun, Nano Energy, 2013, 2, 394–402 CrossRef CAS PubMed.
- H. Xia, Y. H. Wan, G. L. Yuan, Y. S. Fu and X. Wang, J. Power Sources, 2013, 241, 486–493 CrossRef CAS PubMed.
- J. S. Xu and Y. J. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 4752–4757 CAS.
- H. Xia, M. O. Lai and L. Lu, J. Mater. Chem., 2010, 20, 6896–6902 RSC.
- J. K. Feng, M. O. Lai and L. Lu, Electrochem. Commun., 2011, 13, 287–289 CrossRef CAS PubMed.
- R. Yi, J. K. Feng, D. P. Lv, M. L. Gordin, S. R. Chen, D. W. Choi and D. H. Wang, Nano Energy, 2013, 2, 498–504 CrossRef CAS PubMed.
- W. Li, Y. X. Yin, S. Xin, W. G. Song and Y. G. Guo, Energy Environ. Sci., 2012, 7, 8007–8013 Search PubMed.
- M. J. Aragón, B. León, C. P. Vicente, J. L. Tirado, A. V. Chadwick, A. Berko and S.-Y. Beh, Chem. Mater., 2009, 21, 1834–1840 CrossRef.
- W. A. Ang, N. Gupta, R. Prasanth and S. Madhavi, ACS Appl. Mater. Interfaces, 2012, 4, 7011–7019 CAS.
- M. J. Aragón, C. V. Pérez and J. L. Tirado, Electrochem. Commun., 2007, 9, 1744–1748 CrossRef PubMed.
- F. Zhang, R. H. Zhang, G. M. Liang, J. K. Feng, L. Lu and Y. T. Qian, Mater. Lett., 2013, 111, 165–168 CrossRef CAS PubMed.
- B. Xu, L. Shi, X. W. Guo, L. Peng, Z. X. Wang, S. Chen, G. P. Cao, F. Wu and Y. S. Yang, Electrochim. Acta, 2011, 56, 6464–6468 CrossRef CAS PubMed.
- M. J. Aragón, B. León, C. P. Vicente and J. L. Tirado, J. Power Sources, 2011, 196, 2863–2868 CrossRef PubMed.
- S. Mirhashemihaghighi, B. Leon, C. Perez Vicente, J. L. Tirado, R. Stoyanova, M. Yoncheva, E. Zhecheva, R. Saez Puche, E. M. Arroyo and J. Romero de Paz, Inorg. Chem., 2012, 51, 5554–5561 CrossRef CAS PubMed.
- F. Zhang, R. H. Zhang, J. K. Feng and Y. T. Qian, Mater. Lett., 2014, 114, 115–118 CrossRef CAS PubMed.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, J. Mater. Chem., 2009, 19, 5047–5054 RSC.
- Z. J. Ding, B. Yao, J. K. Feng and J. X. Zhang, J. Mater. Chem., 2013, 1, 11200–11209 RSC.
- L. W. Su, Z. Zhou, X. Bin, Q. W. Tang, D. H. Wu and P. W. Shen, Nano Energy, 2013, 2, 276–282 CrossRef CAS PubMed.
- S. Q. Zhao, Y. Yu, S. S. Wei, Y. X. Wang, C. H. Zhao, R. Liu and Q. Shen, J. Power Sources, 2014, 253, 251–255 CrossRef CAS PubMed.
- Y. R. Zhong, L. W. Su, M. Yang, J. P. Wei and Z. Zhou, ACS Appl. Mater. Interfaces, 2013, 5, 11212–11217 CAS.
- T. Brezesinski, J. Wang, J. Polleux, B. Dunn and S. H. Tolbert, J. Am. Chem. Soc., 2009, 131, 1802–1808 CrossRef CAS PubMed.
- J. W. Morse, R. S. Arvidson and A. Lüttge, Chem. Rev., 2007, 107, 342–381 CrossRef CAS PubMed.
- B. Xu, L. Peng, G. Q. Wang, G. P. Cao and F. Wu, Carbon, 2010, 48, 2361–2380 CrossRef PubMed.
- Q. C. Zhuang, J. Li and L. L. Tian, J. Power Sources, 2013, 222, 177–183 CrossRef CAS PubMed.
- S. M. Paek, E. Yoo and I. Honma, Nano Lett., 2009, 1, 72–75 CrossRef PubMed.
- X. C. Li, X. Y. Xu, F. L. Xia, L. X. Bu, H. X. Qiu, M. X. Chen, L. Zhang and J. P. Gao, Electrochim. Acta, 2014, 130, 305–311 CrossRef CAS PubMed.
- Y. Sharma, N. Sharma, R. G. V. Subba and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2862 CrossRef CAS.
- Z. Chen, Y. Yan, S. Xin, W. Li, J. Qu, Y. G. Guo and W. G. Song, J. Mater. Chem. A, 2013, 1, 11404–11409 CAS.
- D. P. Lv, M. L. Gordin, R. Yi, T. Xu, J. X. Song, Y. B. Jiang, D. W. Choi and D. H. Wang, Adv. Funct. Mater., 2014, 24, 1059–1066 CrossRef CAS.
- H. Xia, D. D. Zhu, Y. S. Fu and X. Wang, Electrochim. Acta, 2012, 83, 166–174 CrossRef CAS PubMed.
- S. X. Jin and C. X. Wang, Nano Energy, 2014, 7, 63–73 CrossRef CAS PubMed.
- K. H. Seng, M. H. Park, Z. P. Guo, H. K. Liu and J. Cho, Nano Lett., 2013, 13, 1230–1236 CrossRef CAS PubMed.
- P. C. Lian, X. F. Zhu, S. Z. Liang, Z. Li, W. S. Yang and H. H. Wang, Electrochim. Acta, 2010, 55, 3909–3916 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1564 CrossRef CAS PubMed.
- T. N. Lambert, C. A. Chavez, B. Hernandez–Sanchez, P. Lu, N. S. Bell, A. Ambrosini, F. Friedman, T. J. Boyle, D. R. Wheeler and D. L. Huber, J. Phys. Chem. C, 2009, 113, 19812–19818 CAS.
- J. Qiu, P. Zhang, M. Ling, S. Li, P. Liu, H. Zhao and S. Zhang, ACS. Appl. Mater. Interfaces, 2012, 7, 3636–3642 Search PubMed.
- W. Li, Y. X. Yin, S. Xin, W. G. Song and Y. G. Guo, Energy Environ. Sci., 2012, 7, 8007–8013 Search PubMed.
- F. Zhang, R. H. Zhang, G. M. Liang, J. K. Feng, L. Lu and Y. T. Qian, Mater. Lett., 2013, 111, 165–168 CrossRef CAS PubMed.
- J. Liu, H. Xia, L. Lu and D. F. Xue, J. Mater. Chem., 2010, 20, 1506–1510 RSC.
- J. Shen, M. Shi, B. Yan, H. Ma, N. Li and M. Ye, J. Mater. Chem., 2011, 21, 7795–7801 RSC.
| This journal is © The Royal Society of Chemistry 2015 |