Development of a Cr(III) ion selective fluorescence probe using organic nanoparticles and its real time applicability†
Anu
Saini
a,
Aman K. K.
Bhasin
a,
Narinder
Singh
*b and
Navneet
Kaur
*a
aCentre for Nanoscience and Nanotechnology (UIEAST), Panjab University Chandigarh, 160014, India. E-mail: navneetkaur@pu.ac.in; Tel: +91-1722534464
bDepartment of Chemistry, Indian Institute of Technology Ropar (IIT Ropar), Rupnagar, Panjab 140001, India. E-mail: nsingh@iitrpr.ac.in; Tel: +91-1881242176
First published on 15th October 2015
Abstract
The use of fluorescent organic nanoparticles for the determination of metal ions is highly significant. Among other analytical techniques, fluorescence spectroscopy finds significance due to high sensitivity, selectivity and ease of operation. A ligand 1 was synthesized using 1,8-naphthalic anhydride and triethylenetetramine and it was further processed into organic nanoparticles that displayed excellent sensing properties for selective detection of Cr3+ ion in aqueous medium using fluorescence spectroscopy. The sensor for Cr3+ ion showed competitive binding as no other metal ions caused interference in detection of Cr3+ ion. To check the efficacy of the sensor, titrations were performed with addition of Cr3+ ion in small aliquots and it efficiently detects Cr3+ ion with a detection limit of 64 nM. The mechanism behind the Cr3+ ion detection was studied using DFT calculations, which showed that chromium gets electrons from the nitrogen part of triethylenetetramine which resulted in inhibition of photoinduced electron transfer (PET) that led to an increase in the fluorescence profile.
Introduction
Nanotechnology has emerged as a revolutionary field in context to its wide applications in many fields. For the past few years, nanomaterials have been showing enormous potential which make them attractive for sensing purposes.1 The fabrication of fluorescent nanoparticles is a popular subject of research as they find extensive applications in interdisciplinary fields.2 Inorganic nanomaterials, like silica nanoparticles and semiconductor quantum dots, find attention in sensing applications. However, these fluorescent inorganic nanoparticles have a shortcoming, as most of these nanomaterials are non-biodegradable. So there are certain toxicity concerns for the utilization of fluorescent inorganic nanoparticles for various applications.3 However fluorescent organic nanoparticles FONs have features different from the quantum dots and polymer nanoparticles, one significant concern is the control of their luminescence properties.4 It has been observed that sometimes during aggregation of the organic compounds, enhancement in the fluorescence profile is observed exhibiting aggregation induced emission enhancement (AIEE) phenomenon. Such organic compounds depict very weak emission in solution; however they display strong fluorescence emission in solid or aggregated form.5 The interchromophoric interactions due to π–π stacking or dimerization lead to quenching in fluorescence intensity during the formation of nanoparticles and the phenomenon is known as aggregation caused quenching (ACQ). There may be the case of non-radiative decay channel which can compete with fluorescence.6 Also the FONs properties are size dependent. The luminescent properties can be controlled by engineering of fluorescent molecules. The electronic properties of the chromophore subunit and interchromophoric interactions need to be monitored.7 Thus fluorescent probes employed for the determination of cations, anions, and other biologically and environmentally important analytes is a matter of great importance. The reason behind the enormous demand for the sensors based on fluorescence phenomena is due to high selectivity, sensitivity and precision. Fluorescent probes based on 1,8-naphthalic anhydride to detect metal ions is achieving consideration due to extensive use of metals in the environment.8 They been comprehensively considered due to their motivating photophysical and biological features and the wide range of their utilities in different chemical and biochemical areas. Naphthalimide derivatives are known as DNA intercalators and display antitumor and anticancer activities. There are naphthalimide scaffolds like amonafide which was in clinical trials but displayed dose-limiting bone marrow toxicity. Mostly, naphthalimide derivatives have not reached the market due to poor therapeutic index and toxic effects.9 Naphthalimide derivatives belong to an extraordinary category of environmentally sensitive fluorophores due to features like high photo stability and their tendency to show high quantum yield.10 Therefore, the naphthalimide moiety has been used extensively for designing probes for quantification and detection of metal ions.11Cr3+ is one of the most vital nutrients for the human body that plays a key role in various biochemical phenomena.12 Cr3+ is vital nutrient in the diet of human beings in the range 50–200 μg per day as per (WHO 1988) reports.13 It improves the insulin sensitivity and works in fats, proteins and carbohydrates metabolism.14 Deficiency of Cr3+ in the diet leads to an increase in risk of diabetes and cardiovascular diseases.15 Also, an excess of Cr3+ causes genotoxic effects. It also acts as pollutant of the environment due to several industrial and agricultural activities.16 Therefore, detection of Cr3+ is of great importance. It has been described as one of the most efficient quenchers among the transition metal series due to its paramagnetic character. There are mostly “turn-OFF” sensors reported in the literature with very few related to fluorescent enhancement in Cr3+.17 Therefore, increase in fluorescence intensity on binding with metal ion is favoured over those exhibiting fluorescence quenching due to sensitivity reasons. Furthermore, the present sensor has been compared with the existing fluorogenic and chromogenic sensors for Cr3+ ion. It has been noticed that for most of the sensors for Cr3+ ion, the medium for studies is an organic–aqueous solvent system. However, in our case the sensor works efficiently in aqueous medium which provides its applicability in environmental samples. Our sensor can detect Cr3+ ion up to a limit of 64 nM, which displays the high sensitivity of this sensor (Table S1, ESI†).
Results and discussion
Synthesis of ligand 1
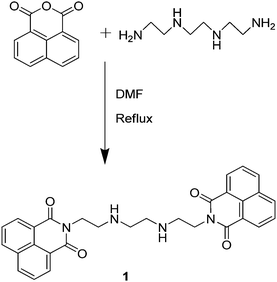
Ligand 1 was synthesized using a literature method18 by taking 1,8-naphthalic anhydride and triethylenetetramine in 15 ml of dimethylformamide (DMF); the reaction mixture was maintained at 80 °C for 12 h (Scheme 1). When the reaction was complete, the reaction mixture was quenched in ice cold water. The solid material was separated out and the product was recrystallized and air dried. Ligand 1 was fully characterized with 1H NMR, IR and mass spectroscopy (Fig. S1–S3, ESI†) and the purity of the sample was checked with elemental analysis and the results were in accordance with reported data.18Fabrication of organic nanoparticles (ONPs) of ligand 1: N1
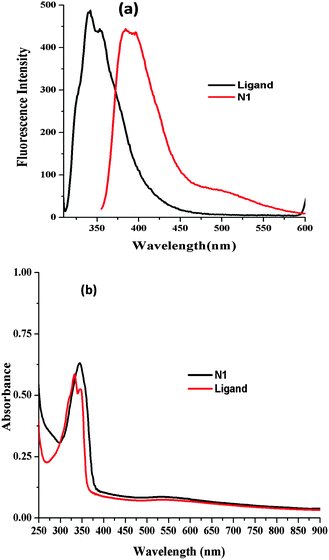
Organic nanoparticles are the particles formed from organic compounds having dimensions of 1–100 nm.19 There is an enormous demand for the formulation of organic nanoparticles for the development of sensors. There are various environmental factors like pH, salt strength and temperature that play a key role to judicially choose the method for fabrication of organic nanoparticles.20 There are different approaches used for the fabrication of organic nanoparticles. In the present work, a single step procedure known as reprecipitation method is utilized for preparing nanoparticles of the ligand in solution which is based on a solvent displacement approach. In the reprecipitation method, the ligand is dissolved in an organic solvent.21 This solution is then slowly injected into a large amount of water. The principal feature behind the formation of organic nanoparticles N1 is the insolubility of the ligand in water. The solubility of ligand 1 was checked in various solvents. However, the best solubility results were obtained with tetrahydrofuran (THF). Then different amounts of ligand 1 dissolved in THF were injected into water and the particle size was regularly monitored using Dynamic Light Scattering (DLS). It was found that when 0.1 ml of ligand was injected into 100 ml of water uniformly sized particles were formed as confirmed by DLS studies. When other compositions of ligand were added to water, it ruined the particle size. The stability of organic nanoparticles N1 was checked over a period of time. The fluorescence spectra were recorded over a period of 7 days and it was noticed that the fluorescence emission profile remained unaffected during this time, thus confirming the stability of the organic nanoparticles (Fig. S4, ESI†).To visualize the consequence of the water environment on the formation of organic nanoparticles, the fluorescence spectrum was recorded for ligand 1 (0.492 μM) in THF, which displayed emission maximum at 340 nm. When the solvent system is changed from THF to water it revealed a bathochromic shift in the emission spectrum with a peak at 395 nm and the development of a new emission band was observed at 500 nm (Fig. 1a). The shift observed in the emission spectra can be explained on the basis of π–π stacking between the naphthyl moieties. Further, the effect of water content on the nanoparticle formation was checked using UV-Visible spectroscopy (Fig. 1b). On changing the solvent system there is bathochromic shift with slight enhancement at 340 nm. The fluorescence emission and absorption profiles for ligand 1 and N1 have been plotted together as shown in Fig. S5 (ESI†).
The uniformity of the size of the organic nanoparticles was studied using particle analyzer DLS studies which revealed that spherical nanoparticles were formed with an average particle size of 18 nm (Fig. 2a). The size of the organic nanoparticles was also checked with the help of Transmission Electron Microscopy (TEM) and the size of the particle came to be around 8 nm (Fig. 2b).
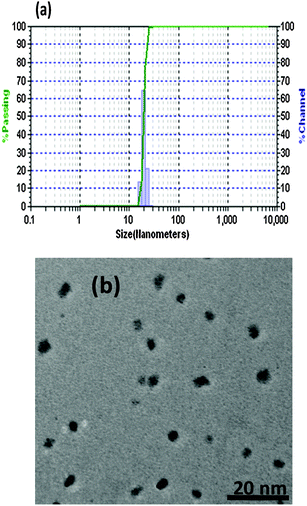 | ||
| Fig. 2 (a) Distribution of particle size of N1 measured with DLS based particle size analyzer showing average particle size of 40 nm; (b) TEM image of organic nanoparticles N1. | ||
Recognition studies with ONP N1
The fluorescence emission studies of ligand 1 (0.492 μM) were carried out in THF to check its selectivity towards different metal nitrate salts. Upon the addition of various metal nitrate salts (20 μM) Li+, Na+, K+, Cs+, Mg2+, Ca2+, Ba2+, Sr2+, Al3+, Cr3+, Mn2+, Fe3+, Co2+, Cu2+, Zn2+, Ag+, Pb2+, Cd2+ and Hg2+ to the ligand solution, the fluorescence emission profile remains unaffected, thus depicting the non-selective behaviour of the ligand solution towards any metal ion (Fig. S6, ESI†).Further, the sensitivity and selectivity of organic nanoparticles N1 (0.492 μM) towards metal ions was investigated using fluorescence emission spectrum on addition of different metal nitrate salts (20 μM) Li+, Na+, K+, Cs+, Mg2+, Ca2+, Ba2+, Sr2+, Al3+, Cr3+, Mn2+, Fe3+, Co2+, Cu2+, Zn2+, Ag+, Pb2+, Cd2+ and Hg2+ to N1. Upon addition of various metal ions there was no or little change in the emission profile; however, when Cr3+ ion was added to N1 the spectrum showed distinct enhancement in fluorescence intensity (Fig. 3). Cr3+ due to its paramagnetic character is known for its quenching ability. The probes based on the quenching properties are not that sensitive or selective. A major drawback of quenching phenomenon based probes is that there can be influence of interfering metal ions and in practical situations the turn-off mechanism is not favourable due to some external factors like change in pH, absorbance of impurities. The enhancement in the emission profile may be attributed to the interaction of the Cr3+ ion with the electron-rich nitrogen of the triethylenetetramine. Due to this interaction ligand molecules are placed in a stable conformation.
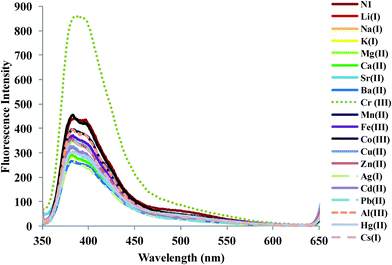 | ||
| Fig. 3 Changes in emission profile of organic nanoparticles N1 (0.492 μM) upon addition of 20 μM of a particular metal ion. | ||
N1 showed a selective linear response in the range of (0–35 μM). The linearity was checked by performing titrations by adding small aliquots of Cr3+ ion to host solution N1 (Fig. 4a). The detection limit was found to be 64 nM which is calculated based on (3.3× σ)/M (see ESI†) where σ is the standard deviation of blank measurements, M is the slope of fluorescence intensity vs. sample concentration plot. The calibration curve for the detection of chromium ion is shown in Fig. 4b.
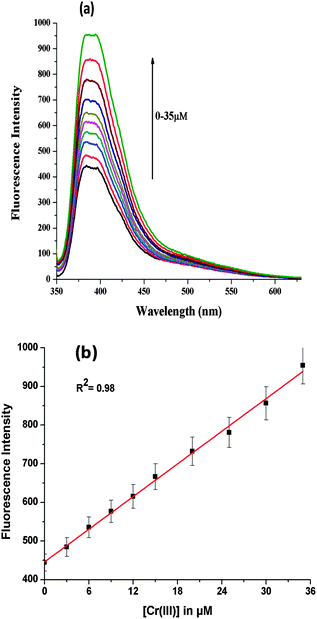 | ||
| Fig. 4 (a) Variation in emission profile of organic nanoparticles N1 (0.492 μM) upon successive addition of Cr3+ ion (0–35 μM); (b) linear regression graph for Cr3+ ion. | ||
Further, the selectivity of N1 was evaluated by observing the effect of other interfering metal ions by measuring the fluorescence intensity in a series of solutions of N1 containing Cr3+ ion. A competitive binding experiment confirmed the selectivity for Cr3+ ion as no other metal ion like Li+, Na+, K+, Cs+, Mg2+, Ca2+, Ba2+, Sr2+, Al3+, Al3+, Mn2+, Fe3+, Co2+, Cu2+, Zn2+, Ag+, Pb2+ and Cd2+ posed any interference (Fig. 5).
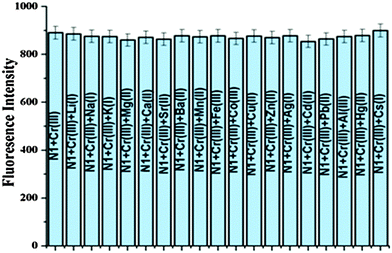 | ||
| Fig. 5 Influence of other metal ions on the Cr3+ ion based variation in the emission profile of organic nanoparticles N1 (0.492 μM). | ||
An increasing amount of perchlorate salt was added to N1 to check the effect of salt on the fluorescence spectrum and it was found that upon addition of 80 equivalents of perchlorate salt the photophysical properties did not change (Fig. S7, ESI†). Also the behaviour of N1 containing Cr3+ was studied as a function of time. The change in the fluorescence spectrum was investigated after short reaction times when different concentrations of Cr3+ ion (16, 24 and 32 μM) were added to N1 (0.492 μM). In the figure, I0 corresponds to the fluorescence intensity of the host solution N1 and the term I corresponds to the fluorescence intensity of solution containing N1 + Cr(III) ion (fixed concentration) at varying times. The results showed that Cr3+ ions interact with N1 within first 20 seconds and after that there is no significant change observed in fluorescence intensity up to 100 seconds (Fig. 6).
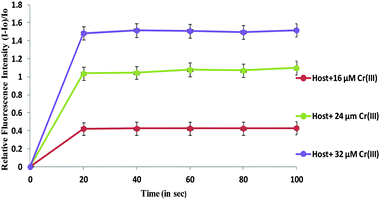 | ||
| Fig. 6 Plot of relative fluorescence intensity (I − I0)/I0 of the nanoparticles and Cr3+ at different concentrations as a function of time in seconds. | ||
DFT calculations for ligand 1
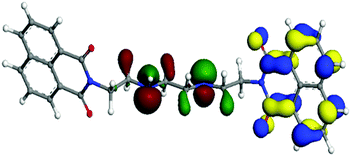
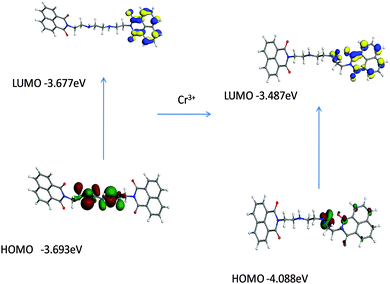
The density functional theory DFT calculations of ligand 1 were performed using GGA-DFT package of DMol3.22 The DFT studies were performed to find the probable interaction site for the cations. The DFT optimized studies revealed that highest occupied molecular orbital (HOMO) was concentrated around nitrogen part of triethylenetetramine in the ligand, while lowest unoccupied molecular orbital (LUMO) was localized on the naphthalimide ring of the ligand (Fig. 7).Further DFT studies were performed to observe the modulations in the photophysical behaviour upon addition of chromium ions into it. It was monitored that when chromium ions were added the HOMO and LUMO of the ligand got stabilized in comparison to their ligand analogues. Upon addition of chromium ion it was noticed that the lone pair gets concentrated on the binding sphere of chromium leading to the stabilization of the ligand (Fig. 8).
Real time analysis
To demonstrate the applicability of the present sensor different types of water samples were analyzed: tap water, lab drainage and river water. The different water samples were spiked with a known concentration of chromium. The sensor performed efficiently in determination of Cr3+ with these water samples without any interference from the other metal ions and displayed noticeable enhancement in emission intensity of N1. For the estimation of concentration present in the samples, the results were compared with the calibration plot. Hence the proposed sensor can be utilized for the determination of Cr3+ ion with different variants of water samples without observing any interference from other metal ions. The results obtained from the sensor for different water samples are listed in Table 1.| S. no. | Water sample | Present sensor (μM) |
|---|---|---|
| 1 | Sample 1 | 16.4 |
| 2 | Sample 2 | 34.1 |
| 3 | Sample 3 | 22.7 |
Conclusion
A naphthalimide-based ligand 1 was synthesized and characterized with NMR and mass spectroscopy. Organic nanoparticles N1 of ligand 1 selectively recognize Cr3+ in aqueous medium. The sensor efficiently detects Cr3+ ion up to a detection limit of 64 nM. DFT studies were performed to explain the change in photophysical behaviour of ligand 1 on addition of chromium and it was concluded that chromium ion causes inhibition of PET phenomenon, as they take away the lone pair from the central nitrogen of triethylenetetramine. Hence, enhancement of fluorescence intensity is observed. The present work was employed for its real time applicability and the results proved that the sensor works exceptionally well for different water samples without depicting any interference from potent interfering metal ions.Experimental
General information
All chemicals purchased from Sigma-Aldrich Co. were of analytical grade. Avance-II (Bruker) instrument, used to record NMR spectra, was operated at 400 MHz for 1H NMR (chemical shifts are expressed in ppm). The UV Visible absorption spectrum was recorded on Spectroscan 30. RF-5301 PC Fluorescence Spectrofluorophotometer was utilized to perform fluorescence studies. IR spectra were recorded on Perkin Elmer-Spectrum RX-IFTIR for the compounds in the solid state as KBr discs or as neat samples in the range 4000 cm−1 to 250 cm−1. For determination of mass of the ligand, LC-MS equipped with a Waters Micromass Q-Tof was used. Hitachi (H-7500) instrument operated at 120 kV recorded TEM images with resolution of 0.36 nm (point to point). The sample was prepared on a 400-mesh formvar carbon-coated grid of copper. Dynamic Light Scattering (DLS) studies to analyze the size of organic nanoparticles were done using external probe feature of Metrohm Microtrac Ultra Nanotrac Particle Size Analyser.Recognition studies
The recognition studies were performed at 25 ± 1 °C. The solutions were shaken and kept for about 30 minutes before recording the spectra. By addition of 20 μM of metal nitrate salts to 5 ml solution taken in volumetric flasks, the binding behaviour of nanoparticles (0.492 μM) of ligand 1 in aqueous medium was determined. For titrations, the metal nitrate salt of Cr3+ was respectively added to volumetric flasks containing organic nanoparticles N1. The possible interference due to different metal cations was checked by the addition of interfering cations (20 μM) to the nanoparticles solution containing Cr3+ ion. The effect of ionic strength was explored by recording the fluorescence profile at different concentration of tetrabutylammonium perchlorate (0–80 equivalent).Synthesis of ligand
Ligand 1
1,8-Naphthalic anhydride (500 mg, 2.52 mmoles) was dissolved in 10 ml DMF and triethylenetetramine (275 μl, 1.832 mmoles) was added to the above solution. The reaction was stirred at 80 °C and refluxed for 12 hours. Progress of the reaction was observed over precoated TLC plate (chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol 9.5
methanol 9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5). The reaction mixture was quenched into ice cold water after completion of the reaction and then filtered. An off-white colored solid was obtained with 70% yield and melting point 190–192 °C. 1H NMR (400 MHz, CDCl3) δ: 8.56 (d, 4H, ArH), 8.17 (d, 4H, ArH), 7.71 (t, 4H, ArH), 4.32 (t, 4H, –CH2), 3.03 (t, 4H, –CH2), 2.87 (s, 4H, –CH2); ESI-MS m/z = 507.2 [M + H]+ where M = C30H26N4O4; IR (KBr, cm−1) 3431 (–NH, str), 3062 (C–H, str, Ar), 2948 (–CH2, str), 2881 (–CH3, str), 1694 (–C
0.5). The reaction mixture was quenched into ice cold water after completion of the reaction and then filtered. An off-white colored solid was obtained with 70% yield and melting point 190–192 °C. 1H NMR (400 MHz, CDCl3) δ: 8.56 (d, 4H, ArH), 8.17 (d, 4H, ArH), 7.71 (t, 4H, ArH), 4.32 (t, 4H, –CH2), 3.03 (t, 4H, –CH2), 2.87 (s, 4H, –CH2); ESI-MS m/z = 507.2 [M + H]+ where M = C30H26N4O4; IR (KBr, cm−1) 3431 (–NH, str), 3062 (C–H, str, Ar), 2948 (–CH2, str), 2881 (–CH3, str), 1694 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, str), 1589 (C
O, str), 1589 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C, str, Ar), 1438 (–CH3, bend), 1343 (C–N, str, Ar); CHN analysis calculated for (C30H26N4O4): C, 71.13; H, 5.1; N, 11.06; found: C, 70.93; H, 5.23; N, 11.12.
C–C, str, Ar), 1438 (–CH3, bend), 1343 (C–N, str, Ar); CHN analysis calculated for (C30H26N4O4): C, 71.13; H, 5.1; N, 11.06; found: C, 70.93; H, 5.23; N, 11.12.
Fabrication of nanoparticles of ligand 1
For the synthesis of organic nanoparticles of ligand 1 reprecipitation was used. The ligand was dissolved in 4 ml of THF and the solution of ligand (1 mg, 0.492 μM) was prepared. Then 0.1 ml of the above solution was slowly injected into 100 ml of water with a micro-syringe. The solution is then sonicated for a total of 20 minutes, making sure the temperature of the solution containing nanoparticles does not rise above 10 °C.Theoretical studies
The geometry of the complex was optimized using DMol3 package with GGA-DFT, using double numerical plus polarization (DNP) as basic set. The local function for the exchange–correlation potential was the BLYP.23 All electrons of the system were treated.Real sample analysis
Real time analysis for the proposed sensor was done for different water samples taken from different water sources (tap water, lab drainage and river water). The samples 1 and 2 were taken from Centre for Nanoscience and Nanotechnology, Sector 25, Panjab University, Chandigarh. Sample 3 was collected from Sutlej River, Ropar, Punjab. For real time application, water samples were collected. These water samples were spiked with known concentrations of chromium ions.Acknowledgements
N. S. is thankful to DST, India, for research funding through research project sanctioned to him (File No. EMR/2014/000613)References
- (a) S. Su, W. Wu, J. Gao, J. Lu and C. Fan, J. Mater. Chem., 2012, 22, 18101 RSC; (b) V. Biju, Chem. Soc. Rev., 2014, 43, 744 RSC; (c) T. Asefa, C. T. Duncan and K. K. Sharma, Analyst, 2009, 134, 1980 RSC.
- (a) X. Zhang, X. Zhang, L. Tao, Z. Chi, J. Xu and Y. Wei, J. Mater. Chem. B, 2014, 2, 4398 RSC; (b) J. Liu, L. Zhang, C. Wang, H. Xu and X. Zhao, Mol. BioSyst., 2010, 6, 954 RSC.
- X. Zhang, X. Zhang, B. Yang, Y. Zhang, M. Liu, W. Liu, Y. Chen and Y. Wei, Colloids Surf., B, 2014, 113, 435 CrossRef CAS PubMed.
- (a) H. B. Fu and J. N. Yao, J. Am. Chem. Soc., 2001, 123, 1434 CrossRef CAS; (b) B. K. An, S. K. Kwon, S. D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410 CrossRef CAS PubMed.
- (a) M. Cai, Z. Gao, X. Zhou, X. Wang, S. Chen, Y. Zhao, Y. Qian, N. Shi, B. Mi, L. Xie and W. Huang, Phys. Chem. Chem. Phys., 2012, 14, 5289 RSC; (b) Y. Zhang, J. Sun, G. Bian, Y. Chen, M. Ouyang, B. Hu and C. Zhang, Photochem. Photobiol. Sci., 2012, 11, 1414 RSC.
- K. Amro, J. Daniel, G. Clermont, T. Bsaibess, M. Pucheault, E. Genin, M. Vaultie and M. B. Desce, Tetrahedron, 2014, 70, 1903 CrossRef CAS.
- (a) L. E. Santos-Figueroa, M. E. Moragues, E. Climent, A. Agostini, R. Martinez-Manez and F. Sancenon, Chem. Soc. Rev., 2013, 42, 3489 RSC; (b) L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993 RSC.
- D. Staneva, I. Grabchev and R. Betcheva, Dyes Pigm., 2013, 98, 64 CrossRef CAS.
- (a) K. J. Kilpin, C. M. Clavel, F. Edafe and P. J. Dyson, Organometallics, 2012, 31, 7031 CrossRef CAS; (b) Z. Chen, X. Liang, H. Zhang, H. Xie, J. Liu, Y. Xu, W. Zhu, Y. Wang, X. Wang, S. Tan, D. Kuang and X. Qian, J. Med. Chem., 2010, 53, 2589 CrossRef CAS PubMed; (c) I. Ott, Y. Xu and X. Qian, J. Photochem. Photobiol., B, 2011, 105, 75 CrossRef CAS PubMed; (d) A. Wu, Y. Xu and X. Qian, Bioorg. Med. Chem., 2009, 17, 592 CrossRef CAS PubMed.
- N. I. Georgiev and V. B. Bojinov, J. Lumin., 2012, 132, 2235 CrossRef CAS.
- (a) J. M. Chovelon and I. Grabchev, Spectrochim. Acta, Part A, 2007, 67, 87 CrossRef PubMed; (b) I. Grabchev, P. Bosch, M. McKenna and D. Stanev, J. Photochem. Photobiol., A, 2009, 201, 75 CrossRef CAS; (c) R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Krugerc and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936 RSC.
- (a) M. Wang, J. Wang, W. Xue and A. Wu, Dyes Pigm., 2013, 97, 47 Search PubMed; (b) Y. Zhou, Y. S. Li, X. L. Tian, Y. Y. Zhang, L. Yang, J. H. Zhang, X. R. Wang, S. Y. Lu, H. L. Re and Z. S. Liu, Sens. Actuators, B, 2012, 161, 1108 CrossRef CAS.
- A. R. Walsh, J. Ohalloran and A. M. Gower, Ecotoxicol. Environ. Saf., 1994, 27, 168 CrossRef CAS PubMed.
- (a) H. Wu, P. Zhou, J. Wang, L. Zhao and C. Duan, New J. Chem., 2009, 33, 653 RSC; (b) Y. J. Lai and W. L. Tseng, Analyst, 2011, 136, 2712 RSC.
- (a) H. Liu, X. Wan, T. Liu, Y. Li and Y. Yao, Sens. Actuators, B, 2014, 200, 191 CrossRef CAS; (b) S. Wu, K. Zhang, Y. Wang, D. Mao, X. Liu, J. Yu and L. Wang, Tetrahedron Lett., 2014, 55, 351 CrossRef CAS.
- F. Hu, B. Zheng, D. Wang, M. Liu, J. Du and D. Xiao, Analyst, 2014, 139, 3607 RSC.
- S. Guha, S. Lohar, A. Banerjee, A. Sahana, I. Hauli, S. K. Mukherjee, J. Sanmartin Matalobos and D. Das, Talanta, 2012, 91, 18 CrossRef CAS PubMed.
- (a) Q. Chen, P. Guo, L. Xu, T. Liu, X. Qian and Q. Yang, Biochimie, 2014, 97, 152 CrossRef CAS PubMed; (b) J. A. Spicer, S. A. Gamage, G. J. Finlay and W. A. Denny, Bioorg. Med. Chem., 2002, 10, 19 CrossRef CAS PubMed.
- J. Allouche, Synthesis of organic and bioorganic nanoparticles: an overview of the preparation methods, 2013, ch. 2, ISBN: 978-1-4471-4212-6 Search PubMed.
- F. Debuigne, L. Jeunieau, M. Wiame and J. B. Nagy, Langmuir, 2000, 16, 7605 CrossRef CAS.
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570 RSC.
- (a) A. Saini, J. Singh, R. Kaur, N. Singh and N. Kaur, New J. Chem., 2014, 38, 4580 RSC; (b) B. Delley, J. Chem. Phys., 2000, 113, 7756 CrossRef CAS.
- (a) S. Patra, D. Maity, A. Sen, E. Suresh, B. Ganguly and P. Paul, New J. Chem., 2010, 34, 2796 RSC; (b) D. K. Hazra, M. Mukherjee, R. Sen, D. Saha, S. Koner and A. K. Mukherjee, J. Mol. Struct., 2013, 1033, 137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data including NMR, mass spectra; photophysical properties including fluorescence spectrum. See DOI: 10.1039/c5nj01843a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |