Nano Fe3O4@APTES@Ni(OH)2 as a catalyst for alcohol oxidation
Pooja B.
Bhat
and
Badekai Ramachandra
Bhat
*
Catalysis and Materials Laboratory, Department of Chemistry, National Institute of Technology Karnataka, Surathkal, Srinivasanagar, 575025, India. E-mail: ram@nitk.edu.in; Fax: +91 824-2474033
First published on 28th October 2014
Abstract
A nanorod shaped nickel hydroxide coated ferrite nanocatalyst was synthesized by a traditional co-precipitation method. The particle size of the nanoferrite was tuned using a variable surfactant ratio to achieve a high surface area. A very high BET surface area (334.55 m2 g−1) was achieved for particles with sizes of 40–130 nm. The superparamagnetic reusable catalyst was found to be active for the selective liquid phase oxidation of alcohols with hydrogen peroxide as a mild oxidant. Nickel hydroxide acted as a Bronsted base working in synergy with the nanoferrite catalyst for alcohol oxidation. The catalytic system was found to catalyse primary and secondary alcohols efficiently (86%) to their corresponding carbonyls in good yields.
Introduction
Environmental concerns have led to the replacement of stoichiometric oxidants with clean and inexpensive molecular oxygen for the selective liquid phase oxidation of alcohols to carbonyl compounds.1,2 In recent years, extensive research has been carried out on nanocatalysis to produce catalysts with low energy consumption and a long life time. Researchers have found magnetic nanoparticles to be attractive candidates for heterogeneous selective aerobic oxidation of alcohols with superior activity and selectivity.3Ferrite has been explored as an economically viable oxidation catalyst with tunable catalytic properties due to the transfer of electrons between Fe2+ and Fe3+ on octahedral sites.4–6 However, ferrite has been considered to be catalytically inactive under mild reaction conditions for the oxidation of alcohols.7 This is due to the very low surface area of 9 m2 g−1 observed in bulk iron oxide. Tuning the particle size of an iron oxide catalyst can enhance its catalytic activity and selectivity.8 Thereby, designing a catalyst with a high surface area is desirable for its application in the oxidation of alcohols. Transition metal hydroxides and mixed hydroxides have been reported as effective heterogeneous catalysts for alcohol oxidation.9 However, tailoring the catalytic activity of nano iron oxide with coordinative transition metal hydroxide groups (basic hydroxide) has been explored less.
Result and discussion
Characterization of the catalyst
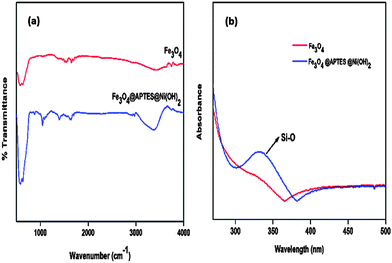
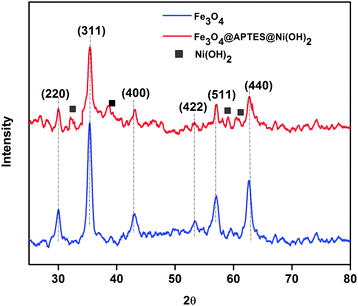
The nickel content in the catalyst was 6 wt% as measured by flame atomic absorption spectroscopy. Fourier Transform Infrared spectroscopy (FTIR) confirmed the aminosilane functionalization of the catalyst (Fig. 1). The basic characteristic peak of Fe3O4 at 580 cm−1 (Fe–O vibration) was observed. The surface hydroxyl groups and physisorbed hydroxyl groups of nickel hydroxide exhibit a broad –OH stretch. Si–O stretching was observed at 1046 cm−1.11 The UV-Visible spectra also confirmed silane adsorption on the catalyst. The appearance of the absorption peak at 350 nm indicated Si–O adsorption on the nanoferrite as shown in Fig. 1b.X-ray Diffraction (XRD) showed major characteristic peaks at 30.0°, 35.4°, 43.2°, 53.6°, 57.2° and 62.0° which can be indexed with the (220), (311), (400), (422), (511) and (400) diffraction planes of cubic spinel structured ferrites (JCPDS 19-0629). The presence of new diffraction peaks observed at 33.0°, 38.8°, 59.6° and 61.05° for Fe3O4@APTES@Ni(OH)2 nanocatalyst confirmed Ni(OH)2 desorption on the ferrite. The diffraction peaks can be indexed with the (100), (101), (110) and (111) diffraction planes of nickel hydroxide, respectively (JCPDS 14-0117). The broad XRD peak confirms the presence of nano sized particles.12 The average crystallite size was calculated from XRD using the Debye Scherer equation using the full width at half maxima (FWHM) of the diffraction peaks.
D = 0.9λ/B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (1) |
The use of 3-aminopropyltriethoxysilane (APTES) as a surfactant introduces highly reactive silanol groups on the surface of the nanoparticles. The morphology of the nanoparticles can be varied by using different amounts of surfactant.13 Hence, the study of ferrite nanoparticles with different concentrations of APTES was examined. APTES acts as a barrier to reduce the interparticle attraction amongst nanosized particles. Thus, the agglomeration tendency amongst nanosized particles can be decreased. Also, the free amine group in APTES assists in the further functionalization of the catalyst. In this study, a uniformly sized nanoparticle with slight agglomeration was observed for naked ferrite (Fig. 4a). As the surfactant concentration was increased, a clustered nanoparticle with a high degree of agglomeration was observed (Fig. 4b and c). A mixture of nanorods and nanoparticles was achieved with a surfactant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 with slight agglomeration (Fig. 4d). The faceted structure obtained can provide active catalytic sites due to its high coordinating ability and large surface area. Hence, further studies on Fe3O4@APTES@Ni(OH)2 nanocatalyst were carried out with a surfactant ratio of 1
0.5 with slight agglomeration (Fig. 4d). The faceted structure obtained can provide active catalytic sites due to its high coordinating ability and large surface area. Hence, further studies on Fe3O4@APTES@Ni(OH)2 nanocatalyst were carried out with a surfactant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. Ageing the nanoparticles with constant stirring may reduce the agglomeration and monodisperse nanosized particles can be achieved. Fig. 5 shows an FEG-SEM image of Fe3O4@APTES@Ni(OH)2 nanocatalyst revealing nanoparticles and nanorods with crystallite sizes of 40 nm and 130 nm.
0.5. Ageing the nanoparticles with constant stirring may reduce the agglomeration and monodisperse nanosized particles can be achieved. Fig. 5 shows an FEG-SEM image of Fe3O4@APTES@Ni(OH)2 nanocatalyst revealing nanoparticles and nanorods with crystallite sizes of 40 nm and 130 nm.
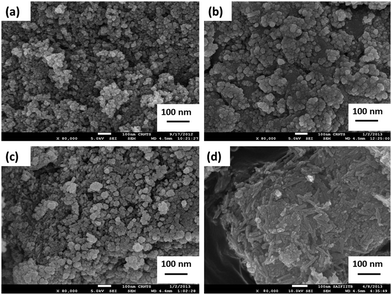 | ||
Fig. 4 FEG-SEM image of (a) naked Fe3O4, (b) Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APTES (1 APTES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (c) Fe3O4 2), (c) Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APTES (1 APTES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (d) Fe3O4 1), (d) Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APTES (1 APTES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) nanocatalysts. 0.5) nanocatalysts. | ||
Transmission Electron Microscope (TEM) images show the dispersion of nickel hydroxide on the spherical and rod shaped ferrites with smooth surfaces (Fig. 6). The average crystallite size observed in FEG-SEM is comparable with that obtained by TEM.
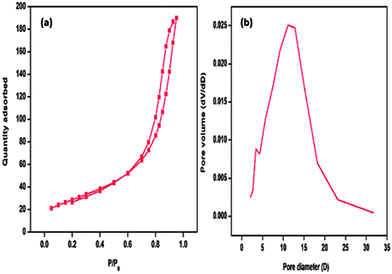
A very high Brunauer–Emmett–Teller (BET) surface area of 334.55 m2 g−1 was achieved for Fe3O4@APTES@Ni(OH)2 nanocatalyst. The nitrogen sorption isotherm (Fig. 7a) exhibits a type-IV isotherm with a sharp inflection at a relative pressure P/P0 of 0.6.14 The hysteresis loop indicates that the catalyst has a mesoporous nature. The Barrett–Joyner–Halenda (BJH) pore size distribution (Fig. 7b) revealed a wide pore size distribution of the catalyst because the pores were derived from a mixture of nanorods and nanoparticles. The pore size distribution adsorption curve of the catalyst was centered at 13 nm.
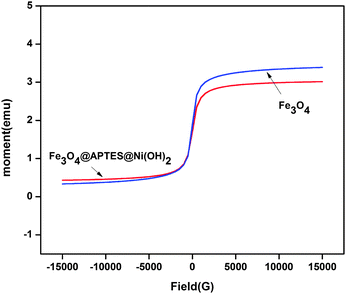
The nanocatalyst exhibited superparamagnetism with very high saturation magnetization MS at room temperature. Magnetic energy loss showed no hysteresis loop with a low coercivity value (16.391 G) as shown in Fig. 8. The adsorption of Ni(OH)2 reduces the saturation magnetisation property of the ferrite. The agglomeration tendency observed in the nanosized particles is reduced due to the superparamagnetic nature of the ferrite. The single domain nanoparticles provide superior magnetic properties, which assist in the instant removal of the nanocatalyst by magnet from the reaction mixture.
Catalytic oxidation of alcohols
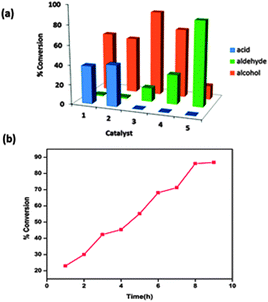
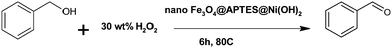
Recently, many researchers have employed ferrite as a catalyst for benzyl alcohol oxidation. Shi et al. observed a maximum 33% conversion with 95% selectivity for benzyl alcohol oxidation with free nano iron oxide.8 Pollshetiwer and Varma tried to overcome this drawback by supporting Pd nanoparticles on dopamine nanoferrite.15 The supported Pd nanoparticles were responsible for the high catalytic activity of the nanocatalyst. Further work was carried out by many researchers employing Au, Ru, Ag metals on the ferrites for improved oxidation of alcohols.16–18 Fe3O4@APTES@Ni(OH)2 nanocatalyst provides an alternative inexpensive catalyst for efficient oxidation of alcohols.Oxidation of alcohols can be accelerated by using a base as a co-catalyst due to its high coordinating ability (Fig. 2).19,20 The oxidation of benzyl alcohol to benzaldehyde was used as model reaction. Product selective liquid phase oxidation of alcohols by different bases has been carried out with the synthesized catalyst (Fig. 9a). It is clear that the use of bases such as KOH and pyridine leads to the formation of acids while the maximum conversion to aldehyde is observed by using Fe3O4@APTES@Ni(OH)2 nanocatalyst. The base facilitated deprotonation of the alcohols, enhancing the catalytic activity. The oxidation of the alcohol with nano Fe3O4@APTES@Ni(OH)2 showed superior catalytic activity with hydrogen peroxide as the terminal oxidant (Table 1). The observed GC conversion is better compared to earlier reports. GC conversion of 86% on the model substrate was observed for the synthesized nanocatalyst. The enhanced catalytic activity observed is due to the high surface area of the nanoparticles, which provides more coordination sites and surface vacancies. The adsorbed Ni(OH)2 on the ferrite nanocatalyst can facilitate deprotonation of the alcohol and provide a synergistic effect on the Lewis center (Fe) of the nanocatalyst.
In this study, optimization of the reaction conditions for alcohol oxidation by the nanocatalyst was carried out, taking into account the influence of solvent, temperature, alcohol/oxidant molar ratio and the length of the reaction time. Optimisation of the reaction conditions using solvents such as acetone, acetonitrile, dichloromethane, toluene and methanol was carried out. The nanocatalyst exhibited a good conversion in acetonitrile with H2O2 as the mild oxidant. Table 2 refers to the optimization of the reaction conditions for the alcohol oxidation. The product analysis was done at regular intervals of time under similar reaction conditions to study the effect of time on the activity (Fig. 9b). It was observed that the GC conversion remains constant after a reaction time of 8 h. The effect of the concentration of the catalyst in the oxidation of alcohols with respect to the model substrate was studied, a significant conversion with 0.08 g of the catalyst was observed (Table 2, entry 6). As the oxidant amount increased the aldehyde conversion, determined by GC, reduced (Table 2, entry 10). This may be due to the overoxidation of alcohols to acids with an increase in the amount of oxidant. The GC conversion observed in the absence of a catalyst was very low (Table 2, entry 1). This revealed the catalytic role of nano iron oxides. GC conversion reduced with an increased catalyst amount (Table 2, entry 7). This may be due to a decrease in the surface area of the nanocatalyst caused by adsorption of oxidized products on the active surface.
| Entry | Catalyst (g) | Amount of oxidant (mmol) | Conversiona (%) |
|---|---|---|---|
| a Reaction conditions: 1 mmol substrate; 10 mmol oxidant (30 wt% H2O2), 3 mL acetonitrile, time (8 h). Conversion determined by GC. | |||
| 1 | 0 | 10.00 | 0.6 |
| 2 | 0.01 | 10.00 | 26.0 |
| 3 | 0.02 | 10.00 | 31.1 |
| 4 | 0.04 | 10.00 | 43.5 |
| 5 | 0.06 | 10.00 | 59.7 |
| 6 | 0.08 | 10.00 | 86.1 |
| 7 | 0.1 | 10.00 | 68.8 |
| 8 | 0.08 | 0 | 4.3 |
| 9 | 0.08 | 5.00 | 39.2 |
| 10 | 0.08 | 15.00 | 64.7 |
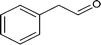
Further, the oxidation was extended to a variety of alcohols using the optimized reaction conditions. The results for the oxidation of a variety of alcohols are summarized in Table 3. Alcohols with an aromatic substituent were found to be more reactive than aliphatic alcohols, which can be attributed to the presence of delocalization. Similarly, electron donating groups were found to slow down the oxidation process whereas electron withdrawing groups accelerate it. Among the various alcohols studied phenylethanol showed the maximum conversion with a GC yield of 98.5%. The enhanced catalytic activity is due to a synergistic effect between the electron donating Ni2+ (Bronsted base) and the Lewis center (Fe) of the nanocatalyst.
| Entry | Substrate | Product | Conversiona (%) |
|---|---|---|---|
| a Substrate (1.0 mmol); 0.08 g catalyst (Ni: 6 wt%); acetonitrile (3.0 mL), oxidant (10 mmol); Ar atm. Conversion and selectivity were determined by GC. Average of 3 GC trials. | |||
| 1 |
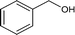
|
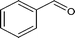
|
86.1 |
| 2 |
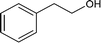
|
|
98.5 |
| 3 |
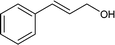
|
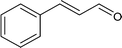
|
96.8 |
| 4 |
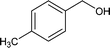
|
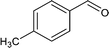
|
68.3 |
| 5 |
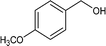
|
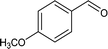
|
66.4 |
| 6 |
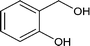
|
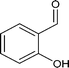
|
64.0 |
| 7 |
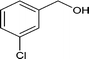
|
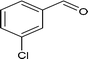
|
70.6 |
| 8 |
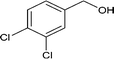
|
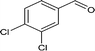
|
76.3 |
| 9 |
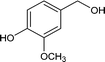
|
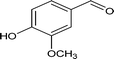
|
73.2 |
| 10 |
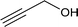
|
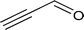
|
40.3 |
| 11 | (CH3)2CH2OH | (CH3)2CH2CHO | 27.6 |
Heterogeneous catalysis and reuse
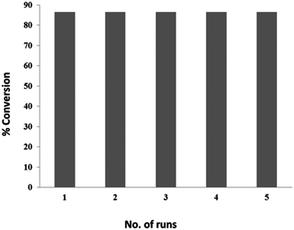
Magnetic catalysts can easily be recovered after the reaction and can be reused without significant loss of catalytic activity and selectivity. A GC conversion of 85% was observed on the model substrate after 5 uses of the catalyst (Fig. 10).Atomic Absorption Spectroscopy (AAS) was carried out in order to verify that the observed oxidation of alcohols was caused by the catalyst or whether it was due to leached nickel species. The adsorbed Ni(OH)2 in the Fe3O4@APTES@Ni(OH)2 nanocatalyst showed a 6 wt% Ni concentration as determined by AAS. This was compared with the Ni concentration of the filtrate obtained during the oxidation reaction. The catalyst was removed using a magnet during heating at about 50% conversion and the reaction mixture was then exposed to the normal reaction conditions for the remaining time. The separated filtrate was further subjected to Atomic Absorption Spectroscopy. The filtrate obtained after the reaction had an Ni concentration of 0.8 wt%. Therefore, the nature of the observed catalysis was truly heterogeneous.21
Conclusions
The following conclusions are drawn from the present study: (a) a superparamagnetic nickel hydroxide functionalized ferrite nanocatalyst was successfully synthesized by a traditional co-precipitation method, (b) nanorod shaped ferrite was achieved by varying the ratio of the surfactant 3-aminopropyltriethoxysilane, (c) the size of the synthesized nanocatalyst was in the range of 40 nm to 130 nm with a surface area of 334.55 m2 g−1. (d) Higher activity was achieved by using the hydroxide coated magnetic nanocatalyst for oxidation of alcohols (a conversion rate of up to 98% was achieved for phenyl ethanol) due to the synergistic effect delivered by nickel hydroxide. (e) A significant number of catalytic cycles is possible, making the catalyst economical. This study offers a promising magnetic nanocatalyst for oxidation of aromatic alcohols to corresponding carbonyls with a high conversion factor by a greener route.Experimental
Synthesis of nanoferrite@APTES
Magnetic Fe3O4 was prepared by a co-precipitation technique. The method involved slow addition of 25% ammonia solution to Fe3O4 nanoparticle precursor solution, FeSO4·7H2O and FeCl3·6H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), under anaerobic conditions at 80 °C. The black precipitate obtained was aged overnight, washed with ethanol and dried at 80 °C.10 Purified Fe3O4 was modified with 3-aminopropyltriethoxysilane (APTES). 1 g of the Fe3O4 nanoparticles was treated with 0.5 g of APTES in the presence of toluene at 80 °C for 6 h in an argon atmosphere. The product was aged for 1 h and centrifuged with toluene, ethanol and dried at 80 °C.
2), under anaerobic conditions at 80 °C. The black precipitate obtained was aged overnight, washed with ethanol and dried at 80 °C.10 Purified Fe3O4 was modified with 3-aminopropyltriethoxysilane (APTES). 1 g of the Fe3O4 nanoparticles was treated with 0.5 g of APTES in the presence of toluene at 80 °C for 6 h in an argon atmosphere. The product was aged for 1 h and centrifuged with toluene, ethanol and dried at 80 °C.
Synthesis of nanoferrite@APTES@nickel hydroxide
The synthesized magnetic Fe3O4 was enveloped with a relatively low amount of nickel hydroxide to enhance the catalytic activity for the oxidation of alcohols. The synthesized Fe3O4 (1 g) was vigorously stirred with an aqueous solution of NiCl2·7H2O (0.6 g) at room temperature for 24 h at pH 13, an aqueous solution of NaOH was then added. The nickel hydroxide functionalized Fe3O4 was washed with deionised water, ethanol and dried at 80 °C.Oxidation reactions
All reactions were carried out in a glass reactor (∼50 mL). Benzyl alcohol (1 mmol), nanoferrite catalyst (0.08 g), acetonitrile (3 mL) were added. H2O2 (30 wt% in water, 10.0 mmol) was added as the oxidant and the reaction mixture was vigorously stirred at 80 °C. Aliquots of the reaction mixture were analyzed by GC. The retention time for different compounds was determined by injecting pure compounds under identical GC conditions.Acknowledgements
The author Miss Pooja thanks NITK, Surathkal for research fellowship, IIT Bombay (CRNTS) for FESEM and TEM analysis. We also thank NIT Trichy for BET surface area analysis.References
- B. Tamami and S. Ghasemi, Appl. Catal., A, 2011, 393, 242 CrossRef CAS PubMed.
- J. Hagen, Industrial Catalysis: A Practical Approach, Wiley-VCH, Weinheim, 1999 Search PubMed.
- C. W. Lim and I. S. Lee, Nano Today, 2010, 5, 412 CrossRef CAS PubMed.
- A. C. Garade, M. Bharadwaj, S. V. Bhagwat, A. A. Athawale and C. V. Rode, Catal. Commun., 2009, 10, 485 CrossRef CAS PubMed.
- J. P. Jacobs, A. Maltha, J. G. H. Reitjes, J. Drimal, V. Ponec and H. H. Brongersma, J. Catal., 1994, 47, 294 CrossRef.
- C. G. Ramankutty and S. Sugunan, Appl. Catal., A, 2001, 218, 39 CrossRef CAS.
- O. Rahman, S. C. Mohapatra and S. Ahmad, Mater. Chem. Phys., 2012, 132, 196 CrossRef CAS PubMed.
- F. Shi, M. K. Tse, M. Pohl, A. Brückner, S. Zhang and M. Beller, Angew. Chem., 2007, 119, 9022 CrossRef.
- Q. Tang, C. Wu, R. Qi, Y. Chen and Y. Yang, Appl. Catal., A, 2011, 403, 136 CAS.
- R. Valenzuela, M. C. Fuentes, C. Parra, J. Baeza, N. Duran, S. K. Sharma, M. Knobel and J. Freer, J. Alloys Compd., 2009, 488, 227 CrossRef CAS PubMed.
- N. T. S. Phan and C. W. Jones, J. Mol. Catal. A: Chem., 2006, 253, 123 CrossRef CAS PubMed.
- V. V. Costa, M. Estrada, Y. Demidova, I. Prosvirin, V. Kriventsov, R. F. Cotta, S. Fuentes, A. Simakov and E. V. Gusevskaya, J. Catal., 2012, 292, 148 CrossRef CAS PubMed.
- F. Shi, M. K. Tse, M. Pohl, J. Radnik, A. Brückner, S. Zhang and M. Beller, J. Mol. Catal. A: Chem., 2008, 292, 28 CrossRef CAS PubMed.
- K. S. W. Singh, Pure Appl. Chem., 1985, 57, 603 Search PubMed.
- V. Polshettiwer and R. S. Varma, Org. Biomol. Chem., 2009, 7, 37 Search PubMed.
- R. L. Oliveira, P. K. Kiyohara and L. M. Rossi, Green Chem., 2012, 12, 144 RSC.
- M. Kotani, T. Koike, K. Yamaguchi and N. Mizuno, Green Chem., 2006, 8, 735 RSC.
- D.-H. Zhang, G.-D. Li, J.-X. Li and J.-S. Chen, Chem. Commun., 2008, 3414 RSC.
- J. Zhu, J. L. Figueiredo and J. L. Faria, Catal. Commun., 2008, 9, 2395 CrossRef CAS PubMed.
- K. Yamaguchi, J. W. Kim, J. He and N. Mizuno, J. Catal., 2009, 268, 343 CrossRef CAS PubMed.
- R. A. Sheldon, M. Wallau, I. W. C. E. Arends and U. Schuchardt, Acc. Chem. Res., 1998, 31, 485 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |