C-dot sensitized Eu3+ luminescence from Eu3+-doped LaF3–C dot nanocomposites†
Tuhin
Samanta
,
Chanchal
Hazra
and
Venkataramanan
Mahalingam
*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER), Mohanpur, Kolkata, West Bengal 741252, India. E-mail: mvenkataramanan@yahoo.com; Fax: +91-33-25873020; Tel: +91-9007603474
First published on 10th November 2014
Abstract
Very strong Eu3+ luminescence is achieved via sensitization by carbon dots (C-dots) in Eu3+-doped LaF3–C-dot nanocomposites for the first time. This energy transfer via C-dots leads to the broadband excitation of Eu3+ ions which can have potential use in phosphor based LEDs.
The lanthanide (Ln3+)-doped nanoparticles have been widely studied because of their sharp electronic transitions occurring within the 4f orbitals. These 4f–4f transitions are forbidden in nature and possess long luminescence lifetimes and narrow emission peaks. These parity forbidden electronic transitions are partly allowed due to mixing of 4f energy states with 5s and 5p orbitals.1–3 The sharp transitions from Ln3+-doped materials have potential applications in phosphors, luminescence displays, light emitting diodes (LEDs), scintillators, bioimaging, etc.4–6 For example, Eu3+-doped Y2O3 is a red phosphor, finding application in the cathode-ray tubes (CRT).7 Similarly, Ce3+-doped YAG is used in phosphor based LEDs.8 However, Ln3+-doped materials suffer from low luminescence quantum efficiency due to their low molar absorptivity
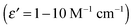 and narrow absorption band.9,10 To increase the absorption cross section of Ln3+ ions some strategies have been developed. One of these strategies is the sensitization of the Ln3+ emission via organic ligands (antenna effect).11–13 In this approach, the organic ligands possessing a high absorption co-efficient are first excited followed by transfer of the excited energy to the Ln3+ ions via the triplet state of the organic molecules.14,15 Another approach is sensitization of Eu3+ emissions through energy transfer from Bi3+ ions which have broad UV excitation. However, energy transfer from Bi3+ doping is generally observed in the solid state and mostly restricted up to 3 or 4% doping.16,17 Thus it is an important challenge to find a strategy to enhance the luminescence efficiency of the Ln3+ ions particularly in water dispersible colloidal materials as they find interesting use in bio-imaging.18 In this work we have developed an approach where carbon dots (C-dots) have been used as a broadband sensitizer for the lanthanide ions such as Eu3+ as well as Tb3+ ions.
and narrow absorption band.9,10 To increase the absorption cross section of Ln3+ ions some strategies have been developed. One of these strategies is the sensitization of the Ln3+ emission via organic ligands (antenna effect).11–13 In this approach, the organic ligands possessing a high absorption co-efficient are first excited followed by transfer of the excited energy to the Ln3+ ions via the triplet state of the organic molecules.14,15 Another approach is sensitization of Eu3+ emissions through energy transfer from Bi3+ ions which have broad UV excitation. However, energy transfer from Bi3+ doping is generally observed in the solid state and mostly restricted up to 3 or 4% doping.16,17 Thus it is an important challenge to find a strategy to enhance the luminescence efficiency of the Ln3+ ions particularly in water dispersible colloidal materials as they find interesting use in bio-imaging.18 In this work we have developed an approach where carbon dots (C-dots) have been used as a broadband sensitizer for the lanthanide ions such as Eu3+ as well as Tb3+ ions.
C-dots are a new class of carbon-based nanomaterials which have unique photoluminescence (PL) properties. They are first obtained during purification of single-walled carbon nanotubes through preparative electrophoresis in 2004.19 Several strategies have been reported for the preparation of C-dots.20–27 The optical properties of the C dots are quite interesting. They show strong excitation dependent PL in the visible region. Though the exact reason for this is debatable, it is generally believed to happen due to a difference in size as well as the presence of several surface related energy states.28–31 Our idea is to explore this broad excitation to enhance the luminescence quantum efficiency of Eu3+ ions in Eu3+-doped LaF3–C dot nanocomposites.
In this communication we report the preparation of Eu3+-doped LaF3–C dot nanocomposites through a hydrothermal process (see the ESI† for more details). Eu3+-doped LaF3–C dot nanocomposites have strong luminescence of C-dots. Upon exciting in a C-dot band (340 nm), strong Eu3+ emission peaks were observed due to energy transfer from C-dots to Eu3+ ions present in LaF3. The importance of the nanocomposites in achieving the energy transfer is studied.
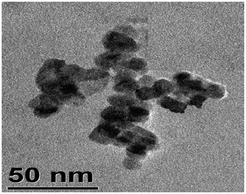
Before preparing the nanocomposites, C-dots were prepared independently by the hydrothermal method and characterized. The X-ray diffraction (XRD) pattern of the C-dots shows a broad peak centered at 2θ = 28° which is characteristic of C-dots.32 The broad nature of the peak implies the amorphous nature of the C-dots. Transmission electron microscopy (TEM) indicates that the average size of the C-dots is about 3–4 nm using triethylene glycol as the carbon source. The TEM image and the XRD pattern of the C-dots are shown in Fig. 1.
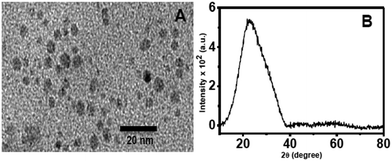 | ||
| Fig. 1 TEM image (A) and XRD pattern (B) of C-dots prepared using triethylene glycol as the carbon source. | ||
The photoluminescence (PL) results of the C-dots are shown in Fig. S1 (ESI†). The C-dots show broad emissions in the region 400 nm and 560 nm when excited between 280 nm and 360 nm. A gradual shift towards longer wavelengths is noted with the increase in the excitation wavelength. These excitation dependent emission maxima are characteristic of C-dots and are mostly related to surface bound energy states.30,31 The lifetime of the C-dots is about 4.02 ns by measuring the emission at 420 nm. The decay curve is shown in Fig. S2 (ESI†).
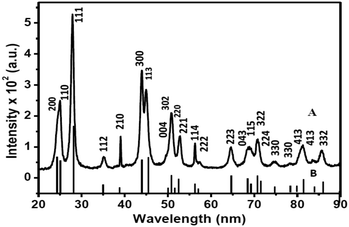
Fig. 2 shows the XRD pattern of Eu3+-doped LaF3–C dot nanocomposites. The pattern clearly indicates the formation of hexagonal LaF3. The peak positions and intensities match well with the standard pattern for pure hexagonal LaF3 crystals (ICSD PDF Card No.; 01-082-0690). The large width of the diffraction peaks indicates the small size of particles. The lattice parameters are a = 7.16 and c = 7.34 Å. The average crystallite size calculated from the (111) diffraction peak of the XRD data based on the Debye–Scherrer formula is 11.5 nm. We emphasize that no characteristic peak of C dots near 2θ = 25° is noticed, which is most likely obscured by the LaF3 peaks.
To understand the shape and size of the Eu3+-doped LaF3 –C dot nanocomposites, a TEM image was taken (shown in Fig. 3). The image indicates the formation of slightly distorted nanocomposites with an average diameter of 13–14 nm. This value is in agreement with that calculated from the Debye–Scherrer formula. We observed that some agglomeration of particles leading to bigger particles was also observed. We emphasized that the presence of C-dots in the nanocrystals is not clear. This might be due to the very small size of the C-dots (3–4 nm) as shown in Fig. 1A. However, energy dispersive X-Ray (EDX) spectroscopy of the nanocomposites shows the presence of C dots. EDX measurements indicate the presence of C, La, F and Eu3+ in the nanocomposites. To verify that the observed carbon peak is indeed due to C dots, we heated the Eu3+-doped LaF3–C dot nanocomposites at 400 °C to remove the C dots. The resulting sample shows very less intense carbon peaks, mostly arising from the carbon present in the glass slide (see Fig. S3, ESI†). Further support for the presence of C-dots in the nanocomposites comes from zeta potential measurements. Zeta potential values of Eu3+-doped LaF3–C dot nanocomposites, Eu3+-doped LaF3 and free C dots are −18.7 mV, 5 mV and −13 mV at pH 7, respectively. These results indicate the presence of C-dots in the nanocomposites and also suggest the stability of nanocomposites in water. In addition, UV absorbance peaks of the nanocomposites show a characteristic peak which is similar to the C dots absorbance spectra reported in the literature (see Fig. S4, ESI†).33
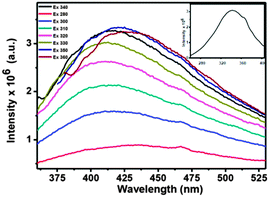
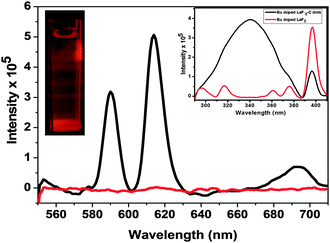
The PL studies confirm the presence of C-dots in the nanocomposites. Fig. 4 shows the PL spectrum of the water dispersed Eu3+-doped LaF3–C dot nanocomposites at different excitation wavelengths from 280 to 360 nm. Strong C-dot emission peaks are observed from 360 to 550 nm when excited between 280 and 360 nm. They show maximum emission intensity at 420 nm when excited at 340 nm. The corresponding excitation spectrum (see the inset of Fig. 4) indicates excitation maxima at 340 nm. In addition, strong Eu3+ emission peaks are observed near 590 and 612 nm upon C-dot excitation (i.e. at 340 nm). These transitions are assigned to 5Do → 7F1 and 5Do → 7F2 transitions, respectively. The PL spectrum of the nanocomposites is shown in Fig. 5 along with the PL spectrum of Eu3+-doped LaF3 nanoparticles. The strong Eu3+ emissions from the nanocomposites are believed to occur via energy transfer from C-dots. This is verified from the excitation spectrum of the nanocomposites which shows a broadband characteristic of C-dots along with sharp peaks from Eu3+ due to 5L0 ← 7F0 (see the inset of Fig. 5). In addition, under identical conditions the PL analysis of Eu3+-doped LaF3 nanoparticles shows only very weak Eu3+ emission intensity (see Fig. 5). This strongly suggests that the strong Eu3+ emissions at 340 nm excitation are due to C-dot sensitization.
We believe the energy transfer from the C-dots to the Eu3+-doped LaF3 is likely of the FRET type. This is supported by the overlap between the emission spectrum of the C-dots and the excitation spectrum of the Eu3+-doped LaF3 (shown in Fig. S5, ESI†). We emphasize that the emission intensity of Eu3+ ions in the nanocomposites upon 340 nm excitation is more intense compared to 394 nm excitation (see Fig. S6, ESI†). The C-dot sensitization of the Eu3+ ions is further verified using lifetime measurements. The lifetimes were measured by exciting the sample at 340 nm and monitoring the emission at 612 nm. This value is 0.32 ms for the Eu3+-doped LaF3–C dot nanocomposites, whereas it is about 0.27 ms for the Eu3+-doped LaF3 nanoparticles at 394 nm excitation. The results are shown in Fig. S7 (ESI†). To evaluate the energy transfer efficiency, we measured the donor (C-dots) lifetime with and without the acceptor (nanocomposites) using the equation 1 − τ0/τ where τ is the lifetime of the C-dot emission in Eu3+-doped LaF3–C dot nanocomposites and τ0 is the lifetime of the C-dots. The values of τ and τ0 are 3.6 ns and 4.02 ns respectively. The calculated energy transfer efficiency is about 11%. The lifetime decay curves of both C-dot emission and the same from nanocomposites are shown in Fig. S8 (ESI†). Though this efficiency seems to be low, it is good as lanthanide ions generally possess low absorption efficiency.
To understand the importance of the nanocomposite formation in the energy transfer, we performed the following control experiment. A physical mixture of Eu3+-doped LaF3 nanoparticles and C-dots was prepared by simple mixing the corresponding dispersions. This physical mixture shows strong C-dots emission and only very weak Eu3+ emission upon excitation at 340 nm. This suggests that formation of nanocomposites is important to observe C-dot sensitized Eu3+ emission (see Fig. S9, ESI†). Furthermore, a simple mixture containing the C-dots and Eu(NO)3 in water shows only an intense C-dot emission (see Fig. S10, ESI†). All these imply the importance of doping Eu3+ ions in a suitable matrix and the formation of nanocomposites.
We have extended a similar study to Tb3+ ions by preparing Tb3+-doped LaF3–C dot nanocomposites under similar experimental conditions. The PL study shows strong Tb3+ emission upon C-dot excitation with a background emission from C-dots. The PL spectrum of Tb3+-doped LaF3–C dot nanocomposites is shown Fig. S11 (ESI†) along with Tb3+-doped LaF3 nanoparticles. Here again the C-dot sensitization leads to higher Tb3+ emission intensity compared to Tb3+-doped LaF3 nanocrystals alone. Extending the study to other Ln3+ ions such as Dy3+ and Tm3+ is obscured by strong C-dot emission in their emission region.
In conclusion we reported the hydrothermal synthesis of Eu3+-doped LaF3–C dot nanocomposites and observed very strong Eu3+ luminescence via sensitization by carbon dots (C-dots) for the first time. This energy transfer via C-dots leads to broadband excitation. The maxima of this broadband excitation are toward far UV (340 nm) compared to direct excitation of Eu3+ (394 nm) which can have potential use in phosphor based LEDs.
Experimental
In a typical procedure, 15 mL of triethylene glycol (TREG) was added to La(NO3)3 (0.95 mmol) and Eu(NO3)3 (0.05 mmol) and heated at 40 °C with vigorous stirring to make a homogeneous dispersion. After 1 h of stirring, solution was transferred to the Teflon-lined stainless-steel autoclave with a total volume of 100 mL. The autoclave was sealed and maintained at 200 °C for 10 h. After the autoclave was cooled to room temperature naturally, the products were collected by centrifugation, washed several times with absolute ethanol, and then dried in a vacuum desiccator. Supernatant carbon dots (C dots) were also collected after centrifugation and stored at 4 °C.VM thanks the Department of Science and Technology (DST), India, Council for Scientific and Industrial Research (CSIR), and IISER-Kolkata for the funding. TS and CH thank UGC and IISER-K, respectively, for the scholarship.
Notes and references
- (a) R. X. Yan and Y. D. Li, Adv. Funct. Mater., 2005, 15, 763 CrossRef CAS; (b) F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang and X. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed; (c) H. A. Hoppe, Angew. Chem., Int. Ed., 2009, 48, 3572 CrossRef PubMed; (d) F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat. Mater., 2011, 10, 968 CrossRef CAS PubMed.
- (a) S. Cotton, Lanthanide and Actinide Chemistry, John Wiley & Sons, West Sussex, 2006 Search PubMed; (b) J. C. G. Bünzli, Chem. Rev., 2010, 110, 2729 CrossRef PubMed; (c) V. Sudarsan, F. C. J. M. van Veggel, R. A. Herring and M. Raudsepp, J. Mater. Chem., 2005, 15, 1332 RSC; (d) M. Haase and H. Schafer, Angew. Chem., Int. Ed., 2011, 50, 5808 CrossRef CAS PubMed; (e) P. Rahman and M. Green, Nanoscale, 2009, 1, 214 RSC.
- (a) P. Y. Jia, J. Lin and M. Yu, J. Lumin., 2007, 122–123, 134 CrossRef CAS PubMed; (b) C. Feldmann, M. Roming and K. Trampert, Small, 2006, 2, 1248 CrossRef CAS PubMed; (c) H. Goesmann and C. Feldmann, Angew. Chem., Int. Ed., 2010, 49, 1362 CrossRef CAS PubMed; (d) T. Montini, A. Speghini, L. DeRogatis, B. Lorenzut, M. Bettinelli, M. Graziani and P. Fornasiero, J. Am. Chem. Soc., 2009, 131, 13155 CrossRef CAS PubMed.
- (a) Z. L. Wang, Z. W. Quan, P. Y. Jia, C. K. Lin, Y. Luo, Y. Chen, J. Fang, W. Zhou, C. J. O'Connor and J. Lin, Chem. Mater., 2006, 18, 2030 CrossRef CAS; (b) F. Meiser, C. Cortez and F. Caruso, Angew. Chem., Int. Ed., 2004, 43, 5954 CrossRef CAS PubMed; (c) D. B. Barber, C. R. Pollock, L. L. Beecroft and C. K. Ober, Opt. Lett., 1997, 22, 1247 CrossRef CAS.
- (a) J. C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC; (b) J. S. Wang, Y. X. Li, Q. Q. Ge, H. C. Yao and Z. J. Li, Appl. Surf. Sci., 2011, 257, 4100 CrossRef CAS PubMed; (c) A. Kar, A. Datta and A. Patra, J. Mater. Chem., 2010, 20, 916 RSC.
- (a) J. Pichaandi, J. C. Boyer, K. R. Delaney and F. C. J. M. van Veggel, J. Phys. Chem. C, 2011, 115, 19054 CrossRef CAS; (b) J. W. Stouwdam and F. C. J. M. van Veggel, Nano Lett., 2002, 2, 733 CrossRef CAS; (c) A. Patra, C. S. Friend, R. Kapoor and P. N. Prasad, Appl. Phys. Lett., 2003, 83, 284 CrossRef CAS PubMed; (d) H. Sami, A. Maparu, A. Kumar and S. Sivakumar, PLoS One, 2012, 7, 1 Search PubMed; (e) B. S. Naidu, B. Vishwanadh, V. Sudarsan and R. K. Vatsa, Dalton Trans., 2012, 41, 3194 RSC.
- (a) J. Li, X. Li, X. Sun and T. Ishigaki, J. Phys. Chem. C, 2008, 112, 11707 CAS; (b) J. Yang, Z. Quan, D. Kong, X. Liu and J. Lin, Cryst. Growth Des., 2007, 7, 730 CrossRef CAS.
- A. Lakshmanan, R. S. Kumar, V. Sivakumar, P. C. Thomas and M. T. Jose, Indian J. Pure Appl. Phys., 2010, 49, 303 Search PubMed.
- (a) S. Li, X. Zhang, Z. Hou, Z. Cheng, P. Maa and J. Lin, Nanoscale, 2012, 4, 5619 RSC; (b) A. K. Parchur, A. I. Prasad, A. A. Ansari, S. B. Rai and R. S. Ningthoujam, Dalton Trans., 2012, 41, 11032 RSC.
- F. Vetrone, J. C. Boyer and J. A. Capobianco, in The Handbook of Luminescence, Display Materials and Devices, ed. L. S. Rohwer and H. S. Nalwa, AmericanScientific Publishers, Los Angeles, 2003 Search PubMed.
- (a) S. I. Weissman, J. Chem. Phys., 1942, 10, 214 CrossRef CAS PubMed; (b) A. Beeby, R. S. Dickins, S. Faulkner, D. Parker and J. A. G. Williams, Chem. Commun., 1997, 1401 RSC.
- R. van Deun, P. Fias, P. Nockemann, K. van Hecke, L. Van Meervelt and K. Binnemans, Inorg. Chem., 2006, 45, 10416 CrossRef CAS PubMed.
- J. Zhang, C. M. Shade, D. A. Chengelis and S. Petoud, J. Am. Chem. Soc., 2007, 129, 14834 CrossRef CAS PubMed.
- (a) A. M. Cross, P. S. May, F. C. J. M. van Veggel and M. T. Berry, J. Phys. Chem. C, 2010, 114, 14740 CrossRef CAS; (b) J. Zhang, C. M. Shade, D. A. Chengelis and S. Petoud, J. Am. Chem. Soc., 2007, 129, 14834 CrossRef CAS PubMed; (c) Y. Chen, Y. M. Chi, H. M. Wen and Z. H. Lu, Anal. Chem., 2007, 79, 960 CrossRef CAS PubMed.
- L. J. Charbonnïere, J. L. Rehspringer, R. Ziessel and Y. Zimmermann, New J. Chem., 2008, 32, 1055 RSC.
- (a) G. F. Ju, Y. H. Hu, L. Chen, X. J. Wang, Z. F. Mu, H. Y. Wu and F. W. Kang, J. Electrochem. Soc., 2011, 158, J294 CrossRef CAS PubMed; (b) M. N. Luwang, R. S. Ningthoujam, S. K. Srivastava and R. K. Vatsa, J. Am. Chem. Soc., 2011, 133, 2998 CrossRef CAS PubMed; (c) F. Xiao, Y. N. Xue and Q. Y. Zhang, Spectrochim. Acta, Part A, 2009, 74, 498 CrossRef CAS PubMed; (d) Y. Tian, B. J. Chen, R. N. Hua, L. H. Cheng, H. Y. Zhong, J. S. Sun, B. Wang, J. Wan, W. L. Lu and K. W. Jang, J. Nanosci. Nanotechnol., 2010, 10, 1943 CrossRef CAS PubMed.
- (a) C. C. Lin, K. M. Lin and Y. Y. Li, J. Lumin., 2007, 126, 795 CrossRef CAS PubMed; (b) Y. C. Kang, S. B. Park, I. W. Lenggoro and K. Okuyama, J. Phys. Chem. Solids, 1997, 60, 379 CrossRef; (c) H. S. Roh, Y. C. Kang and S. B. Park, J. Colloid Interface Sci., 2000, 228, 195 CrossRef CAS PubMed.
- C. Sun, G. Pratx, C. M. Carpenter, H. Liu, Z. Cheng, S. S. Gambhir and L. Xing, Adv. Mater., 2011, 23, H195 CrossRef CAS PubMed.
- X. Y. Xu, R. Ray, Y. L. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736 CrossRef CAS PubMed.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS PubMed.
- (a) S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546 CrossRef CAS; (b) H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473 CrossRef CAS PubMed.
- (a) S. T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi and Y. P. Sun, J. Am. Chem. Soc., 2009, 131, 11308 CrossRef CAS PubMed; (b) W. Kwon and S. Rhee, Chem. Commun., 2012, 48, 5256 RSC.
- (a) S. L. Hu, K. Y. Niu, J. Sun, J. Yang, N. Q. Zhao and X. W. Du, J. Mater. Chem., 2009, 19, 484 RSC; (b) Y. Zhang, H. Gonçalves, J. C. G. Esteves da Silva and C. D. Geddes, Chem. Commun., 2011, 47, 5313 RSC; (c) L. Y. Zheng, Y. W. Chi, Y. Q. Dong, J. P. Lin and B. B. Wang, J. Am. Chem. Soc., 2009, 131, 4564 CrossRef CAS PubMed.
- J. Lu, J. X. Yang, J. Wang, A. Lim, S. Wang and K. P. Loh, ACS Nano, 2009, 3, 2367 CrossRef CAS PubMed.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, V. Georgakilas and E. P. Giannelis, Chem. Mater., 2008, 20, 4539 CrossRef CAS.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455 CrossRef CAS PubMed.
- (a) H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118 RSC; (b) W. Xiaohui, Q. Konggang, X. Bailu, R. Jinsong and Q. Xiaogang, J. Mater. Chem., 2011, 21, 2445 RSC; (c) H. Yan, M. Tan, D. Zhang, F. Cheng, H. Wu, M. Fan, X. Mab and J. Wang, Talanta, 2013, 108, 59 CrossRef CAS PubMed.
- H. Li, X. He, Y. Liu, H. Huang, S. Lian, S. T. Lee and Z. Kang, Carbon, 2011, 49, 605 CrossRef CAS PubMed.
- P. C. Hsu and H. T. Chang, Chem. Commun., 2012, 48, 3984 RSC.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736 CrossRef CAS PubMed.
- F. Wang, Z. Xie, H. Zhang, C. Y. Liu and Y. G. Zhang, Adv. Funct. Mater., 2011, 21, 1027 CrossRef CAS.
- N. Puvvada, B. N. P. Kumar, S. Konar, H. Kalita, M. Mandal and A. Pathak, Sci. Technol. Adv. Mater., 2012, 13, 045008 CrossRef.
- Y. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. Monica Veca and S. Xie, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of Eu3+-doped LaF3–C dot nanocomposites, Eu3+-doped LaF3–C dot nanocomposites, Tb3+-doped LaF3–C dot nanocomposites, Dy3+-doped LaF3–C dot nanocomposites and Tm3+ doped LaF3, TEM, EDAX, the lifetime of nanocomposites, UV-Vis spectra, PL of a physical mixture of Eu3+-doped LaF3 and C dots and PL of Tb3+ doped LaF3–C-dot nanocomposites and Tb3+ doped LaF3. See DOI: 10.1039/c4nj01647e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |