Tough hyperbranched epoxy/neem-oil-modified OMMT thermosetting nanocomposite with an antimicrobial attribute
Bibekananda
De
a,
Kuldeep
Gupta
b,
Manabendra
Mandal
b and
Niranjan
Karak
*a
aAdvanced Polymer and Nanomaterial Laboratory, Department of Chemical Sciences, Tezpur University, Napaam-784028, Assam, India. E-mail: karakniranjan@yahoo.com; Fax: +91-3712-267006
bDepartment of Molecular Biology and Biotechnology, Tezpur University, Napaam-784028, Assam, India. E-mail: mandal@tezu.ernet.in
First published on 24th October 2014
Abstract
In the present study, a high performance, tough, antimicrobial, hyperbranched epoxy nanocomposite is fabricated by the incorporation of neem oil-immobilized organo-modified montmorillonite nanoclay. The immobilization of neem oil with organo-modified montmorillonite nanoclay is performed by the combined effect of mechanical and ultrasonic forces under ambient conditions. This immobilization is confirmed by FTIR and XRD studies. FTIR, XRD, SEM and TEM analyses also confirm the formation of the exfoliated nanocomposite. The dose-dependent enhancements of tensile strength (50%), elongation at break (3-fold), toughness (5.5-fold) and thermal stability (20 °C) of the pristine epoxy thermoset are observed for the nanocomposite. Antimicrobial studies are performed by growth curve and zone-of-inhibition analyses against different bacteria and a fungus at different doses of neem oil-immobilized organo-modified montmorillonite nanoclay. The nanocomposite with 50 wt% neem oil-immobilized organo-modified montmorillonite nanoclay (2.5 wt%) shows significant activity against biofilm formation compared to the pristine thermoset. Thus, the studied nanocomposite has strong potential as a high-performance functional material.
Introduction
Polymer nanocomposites are one of the most active areas of development in the domain of nanotechnology and materials science. The incorporation of nanoclays into various polymers to enhance properties like mechanical, thermal, physical, barrier, have been extensively reported over the last two decades.1–3 The high surface area, favorable intercalation chemistry and natural availability of nanoclays make them the most popular among all the nanomaterials.4,5 They have a high tendency to absorb or intercalate different bio-molecules like drugs or biocides,6,7 organo-molecules8 or different nanoparticles9,10 between their layer structures, and thus recently they have attracted considerable attention in the biological field. However, high moisture absorption and incompatibility with hydrophobic polymers restrain the incorporation of clay particles into the polymer matrix. Thus, organically modified clay is largely used in polymer nanocomposites, and this modification can be performed either by surface functionalization or by the cation exchange process.11–13 The modification of clay is mainly performed to achieve improved properties as well as to obtain a new set of desired properties. Among different matrices for the fabrication of polymer–clay nanocomposites, epoxy resins are extensively used. Furthermore, the design of hyperbranched architecture in the epoxy structure offers a golden feather into it. This is because of their high solubility, low viscosity, and high reactivity, in addition to the fact that thermosets also possess high tensile strength, high modulus, high stiffness, high thermal stability and high chemical resistance.14–16 However, such approaches cannot properly address the main drawback of low toughness or the highly brittle nature of the epoxy thermoset. Thus, this observation prompted researchers to investigate further modification of the conventional OMMT in such a way that the abovementioned problem of epoxy thermoset can be addressed.On the other hand antimicrobial polymer nanocomposites are promising materials in advanced surface coatings for destructing microorganism in different fields including marine industries because of slow release of the active agents. Traditionally, antimicrobial materials contain one or more toxic compounds like biocides and metal nanoparticles, which may cause human health as well as environmental hazards due to leaching problem.17–19 Therefore, a natural antibiotic- or biopesticide-immobilized, clay-reinforced polymer nanocomposite is preferred, although no such report is found, except our earlier report.6 Moreover, the active agent used earlier, H. aromatica, had the problem of volatility and availability. On the other hand, among the different natural biocides, neem (Azadirachta indica) seed oil is a very common and popular agent because of its nontoxic nature to mammals; moreover, it is a very effective antiseptic, antifungal, antibacterial and insecticide.20,21 Crude neem seed oil contains azadirachtin-like biocidal triterpenoid compounds, as well as long chain fatty acids like linoleic acid, oleic acid, palmitic acid, and stearic acid.22,23
Thus, in the present study, crude neem seed oil-immobilized organically modified montmorillonite clay (OMMT) is used for the fabrication of high performance antimicrobial hyperbranched epoxy nanocomposites. Here, neem oil will act as an antimicrobial agent, and the fatty acids of the oil may toughen the whole system by the plasticizing effect. Therefore, the use of hyperbranched epoxy is of particular interest because of its easy synthetic accessibility, low viscosity, high solubility and a large number of end functional groups. In this study, OMMT helps in enhancing the performance of the matrix as well as providing stability to the active agent.
Experimental
Materials
The hyperbranched epoxy resin used in this study was prepared from bisphenol-A, triethanol amine and epichlorohydrin by a polycondensation reaction as previously reported.14 The epoxy equivalent, degree of branching and viscosity of the prepared hyperbranched epoxy were 358 g per eq., 0.79 and 19 Pa s (at 25 °C), respectively. Octadecyl amine modified montmorillonite nanoclay (OMMT) was purchased from Sigma-Aldrich, Germany. Crude neem seed oil was acquired from Agri Life, India, and used after vacuum drying. Poly(amido-amine) hardener (HY840, amine value 5–7 eq. per kg) was obtained from Ciba Giegy, India. Tetrahydrofuran (THF, Merck, India) was used after distillation, and all other chemicals used in this study were of reagent grade.Immobilization of neem oil on OMMT
Crude neem oil (NO) was immobilized on OMMT by using the effect of mechanical and ultrasonic forces at room temperature. In a typical process, 0.5 g OMMT was dispersed in 25 mL THF by magnetic stirring for 30 min in a 60 mL glass bottle. 20 wt% of crude neem oil (0.1 g) was added into it and stirred continuously for 5 h, followed by ultrasonication for 10 min at room temperature. The neem oil-immobilized OMMT was coded as NO–OMMT. In order to study the effect of excess neem oil, OMMT was immobilized with 50 wt% neem oil (with respect to OMMT) and coded as ENO–OMMT.Preparation of hyperbranched epoxy/NO–OMMT nanocomposites
The hyperbranched epoxy/NO–OMMT nanocomposites were prepared by a solution technique.11,24 Three different weight percentages (1, 2.5 and 5) of NO–OMMT dispersed in THF were incorporated into the hyperbranched epoxy and stirred magnetically for 5 h at room temperature, followed by sonication for 10 min by an ultrasonic processor (UP200S, Hielscher, Germany) with a standard sonotrode (tip diameter, 3 mm) at 60% amplitude and 0.5 cycles with an acoustic power density of 460 W cm−2. An amount of 50 wt% (with respect to hyperbranched epoxy) poly(amido-amine) was mixed homogeneously with the abovementioned mixtures and coated on glass and steel plates. The plates were maintained for 24 h under vacuum at room temperature to remove THF and other volatiles. Initially (when the samples were in a low viscous state), high vacuum was applied to remove THF, and the coated plates were then maintained on a flat platform to obtain even surface of the cast film. Then, the samples were placed again under controlled vacuum, and two consecutive weightings after 24 h and 30 h of the abovementioned coated plates were taken to confirm the complete removal of THF or other volatiles. During this period, just gelation of the samples was started, and no significant crosslinking was observed (swelling value, 72%). Finally, the plates were cured inside a furnace at 100 °C for 60 min, followed by post-curing at 130 °C for 30 min. The nanocomposites were coded as PNC1, PNC2.5 and PNC5 for 1, 2.5 and 5 wt% neem oil-modified OMMT, respectively. Similarly, a hyperbranched epoxy nanocomposite with 2.5 wt% unmodified OMMT was prepared (coded as PC2.5) for comparison. The pristine hyperbranched epoxy thermoset was coded as P. In order to study the effect of excess neem oil, a 2.5 wt% ENO–OMMT-based nanocomposite was prepared and coded as PENC2.5.Characterization
FTIR spectra were recorded on a Nicolet FTIR spectrometer (Impact-410) using a KBr pellet. The wide-angle X-ray diffraction patterns of the nanomaterials and the nanocomposites were recorded by a Miniflex (Rigaku Corporation, Japan) X-ray diffractometer using CuKα radiation (0.154 nm). The morphology of the hyperbranched epoxy/NO–OMMT nanocomposites was studied by high-resolution transmission electron microscopy (HRTEM, JEOL, JEMCXII, transmission electron microscope, Japan, operating at 200 kV). The tensile strength of the pristine thermoset and the nanocomposite films (size: 60 × 10 × 0.3 mm3) were measured by universal testing machine (UTM WDW10, China) with a 500 N load cell at a crosshead speed of 10 mm min−1 using the standard test ASTM D822. A scratch hardness test (ASTM G171) was performed using a scratch hardness tester (Sheen Instrument Inc. Ltd, UK) on the surface of the glass-coated thermoset films (size: 75 × 25 × 0.3 mm3). Impact testing was carried out by an impact tester (S. C. Dey & Co., India) as per the standard falling ball method (ASTM D1709) using the steel plate-coated thermoset films (size: 150 × 50 × 0.3 mm3). The bending test of the thermoset films was carried out by the ASTM D522 method using a mandrel with a diameter 1–100 mm. All of the tests for the measurement of mechanical properties were repeated five times and average values were taken. The thermal stability of the thermosets was measured by thermogravimetric analysis (PerkinElmer TG4000) with a nitrogen flow rate of 30 mL min−1 and a heating rate of 10 °C min−1 from 30 to 700 °C. The chemical resistance test was carried out in various chemical environments like aqueous NaOH (5%), aqueous HCl (10%), aqueous NaCl (20%), aqueous ethanol (20%) and tap water to investigate the effects of these chemicals on the films of a pristine hyperbranched epoxy thermoset and its nanocomposite. The films were cut into small pieces (1 cm2, 0.1–0.15 g) and maintained in 100 mL amber bottles containing the abovementioned media at an ambient temperature (25 °C). The percentage of weight loss of the films was measured after 15 days of testing.Antimicrobial study of the nanocomposites
Antimicrobial tests were performed by a well diffusion method as reported in previous studies.25,26Staphylococcus aureus (MTCC 3160) and Bacillus subtilis (MTCC 121) as gram positive bacterial strains; Klebsiella pneumoniae (MTCC 618) and Pseudomonas diminuta (MTCC 3361) as gram negative bacterial strains and Candida albicans (MTCC 3017) as a fungal strain were used in the antimicrobial assay. 200 μL of a log phase culture of the test microbes was seeded on the surface of the Muller-Hinton agar (potato dextrose agar for fungal study) on Petri dishes. The nanocomposites were dispersed in sterilized DMSO, and 100 μL of them were placed into 6 mm diameter wells. In one well, DMSO was taken as a blank, and in another well, Gentamicin (Nystatin for fungal), was used as a positive control. The zone of inhibition diameters were measured using a transparent ruler after incubation for 24 h at 37 °C (for bacteria) and for 48 h at 28 °C (for fungus). For growth curve analysis of the microbes, the cultures were taken in conical flasks. 200 μL of the samples were added to the corresponding conical flasks and incubated for 24 h at 37 °C (for bacteria) and for 48 h at 28 °C (for fungus). One conical flask without the sample was taken as the control for testing each microbe. The growth of the microbes was measured by checking optical density (OD) at 620 nm after every 2 h, and the OD was taken up to 20 h.Biofilm formation study
Biofilm formation on P and PENC2.5 was studied by means of a microtiter plate biofilm assay.27,28 In the present study, a slight modification was performed; herein, direct thermoset films (size 1 cm2) were used instead of microtiter plates. The films were incubated for 72 h in a potato dextrose broth (PDB) medium. The films were gently washed thrice with phosphate buffer saline (PBS, pH 7.4) to remove the planktonic bacteria. Then, the films were resuspended and homogenized in PBS by rigorous vortexing for 5 min, and the cells were serially diluted and plated onto PDB agar. Finally, colony-forming units (CFU) were enumerated after 48 h of incubation at 28 °C.Results and discussion
Formation and characterization of neem oil-immobilized OMMT
Neem oil-immobilized OMMT was formed by combining the effect of mechanical shearing and ultrasonication forces. These forces help in the dispersion of OMMT and the interaction of its layers by neem oil due to the presence of polar groups in both. The immobilization of neem oil into OMMT was first characterized by a FTIR study (Fig. 1). In FTIR spectra, the following bands were found: for neem oil νmax (cm−1): 3472 (–OH), 2928 (–CH), 1750 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ester) and 1458 (C–O, ester); for OMMT: 3640 (–NH), 3414 (–OH), 2928 (–CH), 1037 (Si–O), 527 (Al–O–Si) and 462 (Si–O–Si);6,10 and in case of NO–OMMT νmax (cm−1): 3627 (–NH), 3427 (–OH), 2928 (–CH), 1736 (C
O, ester) and 1458 (C–O, ester); for OMMT: 3640 (–NH), 3414 (–OH), 2928 (–CH), 1037 (Si–O), 527 (Al–O–Si) and 462 (Si–O–Si);6,10 and in case of NO–OMMT νmax (cm−1): 3627 (–NH), 3427 (–OH), 2928 (–CH), 1736 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ester), 1627 (C
O, ester), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, amide), 1471 (C–O, ester), 1037 (Si–O), 527 (Al–O–Si) and 462 (Si–O–Si). The shifting of the ester linkages (C
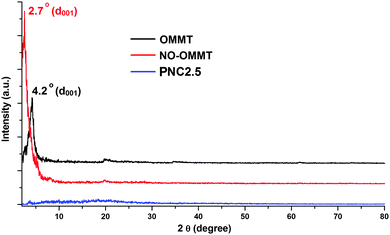
O, amide), 1471 (C–O, ester), 1037 (Si–O), 527 (Al–O–Si) and 462 (Si–O–Si). The shifting of the ester linkages (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O) of neem oil from 1750 and 1458 cm−1 to 1736 and 1471 cm−1 in NO–OMMT reveals the presence of different interactions like hydrogen bonding and different polar–polar interactions, of the neem oil with the OMMT clay. The presence of amide linkage in NO–OMMT along with ester linkage confirms that few ester groups of neem oil chemically interact with the amine groups (octadecyl amine) of OMMT. The interaction of neem oil with the clay galleries was also confirmed by XRD study (Fig. 2). In the XRD patterns, the basal peak (d001) of OMMT was shifted from 2θ = 4.2° to 2.7° after the immobilization of neem oil. Thus, layer spacing increased by 1.17 nm after immobilization, as can be calculated from XRD data using Bragg’s equation. This shifting confirmed that neem oil is immobilized into the clay galleries, and the fatty ester chains of neem oil intercalated into the layers by interacting with them.
O and C–O) of neem oil from 1750 and 1458 cm−1 to 1736 and 1471 cm−1 in NO–OMMT reveals the presence of different interactions like hydrogen bonding and different polar–polar interactions, of the neem oil with the OMMT clay. The presence of amide linkage in NO–OMMT along with ester linkage confirms that few ester groups of neem oil chemically interact with the amine groups (octadecyl amine) of OMMT. The interaction of neem oil with the clay galleries was also confirmed by XRD study (Fig. 2). In the XRD patterns, the basal peak (d001) of OMMT was shifted from 2θ = 4.2° to 2.7° after the immobilization of neem oil. Thus, layer spacing increased by 1.17 nm after immobilization, as can be calculated from XRD data using Bragg’s equation. This shifting confirmed that neem oil is immobilized into the clay galleries, and the fatty ester chains of neem oil intercalated into the layers by interacting with them.
Formation and characterization of hyperbranched epoxy/NO–OMMT nanocomposites
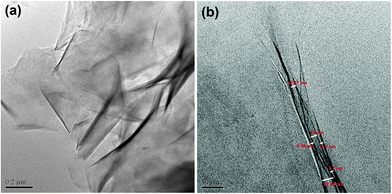
The hyperbranched epoxy/NO–OMMT nanocomposites were prepared by a solution technique using the combined effect of mechanical shearing force and ultrasonication. The compositions of the components in the nanocomposites are given in Table 1. The prepared nanocomposites were characterized by FTIR, XRD, SEM and TEM analyses. In the FTIR spectrum of PNC2.5, the bands were found at νmax (cm−1): 3400 (–OH), 2928 (–CH), 1640 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, amide), 1458 (C–O), 1037 (Si–O), 565 (Al–O–Si) and 462 (Si–O–Si). Herein, the shifting of –OH and Al–O–Si bands of NO–OMMT from 3427 to 3400 and from 527 to 565 cm−1 after formation of the nanocomposite is due to the presence of different interactions such as hydrogen bonding, polar–polar, etc., of NO–OMMT with the hyperbranched epoxy and the poly(amido-amine) hardener.11 In the nanocomposite, the ester linkage of NO–OMMT at 1736 cm−1 completely vanished, whereas only amide linkage was found at 1640 cm−1. This confirmed that all of the ester linkages of NO–OMMT are chemically reacted with the hardener during the curing time and converted into amide linkages. In the XRD pattern, d001 diffraction peak of NO–OMMT at 2θ = 2.7° completely vanished after the formation of the nanocomposite (PNC2.5), as shown in Fig. 2. This may be due to the strong interactions of the polymer chains with clay galleries, which cause complete delamination of the clay layers. It may also happen due to the masking effect of the clay with the polymer as the amount of clay was very less (2.5 wt%). The TEM images (Fig. 3) disclose the actual picture of the structure of the nanocomposites. The images reveal the disordered exfoliated structure of the NO–OMMT layers in the hyperbranched epoxy matrix. This disordered exfoliated structure is due to the strong physicochemical interactions of the chains of hyperbranched epoxy with NO–OMMT galleries, which give uniform dispersion of the platelets in the matrix. The exfoliated interlayer spacing of the nanocomposite is shown in Fig. 3(b), in which we can see that interlayer distance between the two clay layers increases up to 8 nm. The strong dispersion of the NO–OMMT clay in the hyperbranched epoxy matrix was found from the SEM image of the fracture surface of the nanocomposite. From the SEM images, it can be seen that the surface of the nanocomposite, PNC2.5 (Fig. 4b), is rougher than the pristine hyperbranched epoxy, P (Fig. 4a). This indicates that the crack propagation will be less in rougher surface from the planner manner as the crack tip is distorted by clay platelets, and thus crack propagation will be more difficult.29
O, amide), 1458 (C–O), 1037 (Si–O), 565 (Al–O–Si) and 462 (Si–O–Si). Herein, the shifting of –OH and Al–O–Si bands of NO–OMMT from 3427 to 3400 and from 527 to 565 cm−1 after formation of the nanocomposite is due to the presence of different interactions such as hydrogen bonding, polar–polar, etc., of NO–OMMT with the hyperbranched epoxy and the poly(amido-amine) hardener.11 In the nanocomposite, the ester linkage of NO–OMMT at 1736 cm−1 completely vanished, whereas only amide linkage was found at 1640 cm−1. This confirmed that all of the ester linkages of NO–OMMT are chemically reacted with the hardener during the curing time and converted into amide linkages. In the XRD pattern, d001 diffraction peak of NO–OMMT at 2θ = 2.7° completely vanished after the formation of the nanocomposite (PNC2.5), as shown in Fig. 2. This may be due to the strong interactions of the polymer chains with clay galleries, which cause complete delamination of the clay layers. It may also happen due to the masking effect of the clay with the polymer as the amount of clay was very less (2.5 wt%). The TEM images (Fig. 3) disclose the actual picture of the structure of the nanocomposites. The images reveal the disordered exfoliated structure of the NO–OMMT layers in the hyperbranched epoxy matrix. This disordered exfoliated structure is due to the strong physicochemical interactions of the chains of hyperbranched epoxy with NO–OMMT galleries, which give uniform dispersion of the platelets in the matrix. The exfoliated interlayer spacing of the nanocomposite is shown in Fig. 3(b), in which we can see that interlayer distance between the two clay layers increases up to 8 nm. The strong dispersion of the NO–OMMT clay in the hyperbranched epoxy matrix was found from the SEM image of the fracture surface of the nanocomposite. From the SEM images, it can be seen that the surface of the nanocomposite, PNC2.5 (Fig. 4b), is rougher than the pristine hyperbranched epoxy, P (Fig. 4a). This indicates that the crack propagation will be less in rougher surface from the planner manner as the crack tip is distorted by clay platelets, and thus crack propagation will be more difficult.29
| Composition (parts) | P | PC2.5 | PNC1 | PNC2.5 | PNC5 | PENC2.5 |
|---|---|---|---|---|---|---|
| Epoxy | 100 | 100 | 100 | 100 | 100 | 100 |
| Poly(amido-amine) | 50 | 50 | 50 | 50 | 50 | 50 |
| OMMT | 0 | 2.5 | 0.83 | 2.08 | 4.17 | 1.67 |
| Neem oil | 0 | 0 | 0.17 | 0.42 | 0.83 | 0.83 |
 | ||
| Fig. 4 SEM images of the fracture surface of (a) pristine hyperbranched epoxy thermoset (P) and (b) nanocomposite (PNC2.5). | ||
Curing study
The curing study of the hyperbranched epoxy resin and all of its nanocomposites was performed with 50 wt% poly(amido-amine) hardener at 100 °C for 1 h, followed by post curing at 130 °C for 30 min. The swelling value of pristine thermoset, as well as its nanocomposites, was checked in THF at room temperature (25 °C). From Table 2, it can be found that the swelling value of P decreased after the formation of nanocomposites both with unmodified OMMT (PC2.5) and NO–OMMT at lower amounts (PNC1). However, the swelling values of the nanocomposites increased at higher amounts of NO–OMMT (PNC5) and immobilized neem oil (PENC2.5). This may be due to the fact that at higher amounts of NO–OMMT or immobilized neem oil, the fatty ester of neem oil provides a plasticizing effect and thus large free volume to the system, which may help to penetrate the solvent molecules.| Parameter | P | PC2.5 | PNC1 | PNC2.5 | PNC5 | PENC2.5 |
|---|---|---|---|---|---|---|
| a Calculated by integrating the area under stress–strain curves. b Instrument limit of the scratch hardness was 10.0 kg (highest). c Instrument limit of the impact strength was 100 cm (highest). d Instrument limit of the mandrel diameter was 1 mm (lowest). | ||||||
| Swelling value (%) | 24 ± 1.0 | 21 ± 0.4 | 22 ± 0.8 | 24 ± 0.2 | 29 ± 0.6 | 34 ± 1.1 |
| Tensile strength (MPa) | 40 ± 1 | 57 ± 1 | 48 ± 4 | 62 ± 3 | 56 ± 2 | 46.5 ± 2 |
| Elongation at break (%) | 18.5 ± 0.5 | 19 ± 1.5 | 43 ± 2 | 56 ± 5 | 69 ± 4 | 88 ± 4 |
| Toughnessa (MPa) | 540 | 834 | 1637 | 2994 | 2680 | 3323 |
| Scratch hardnessb (kg) | 9.0 ± 0.5 | 10.0 | >10.0 | >10.0 | >10.0 | 10.0 |
| Impact resistancec (cm) | >100 | >100 | >100 | >100 | >100 | >100 |
| Bending diameterd (mm) | <1 | <1 | <1 | <1 | <1 | <1 |
| Initial degradation temperature (°C) | 267 | 282 | 278 | 285 | 288 | 279 |
Mechanical properties of the nanocomposites
The mechanical properties of the pristine hyperbranched epoxy thermoset and its nanocomposites with unmodified and neem oil modified OMMT are given in Table 2. Herein, more than 50% increment of tensile strength and more than 3-fold increment of elongation at break were found for the pristine hyperbranched epoxy thermoset after the formation of the nanocomposite with 2.5 wt% of NO–OMMT. However, a 40% increment of tensile strength and no change in elongation at break were observed after the formation of the nanocomposite with 2.5 wt% of unmodified OMMT. Thus, the increment of toughness, i.e. the area under stress–strain curves (Fig. 5) of hyperbranched epoxy thermoset, was much higher in PNC2.5 (2994 MPa) than PC2.5 (834 MPa). This is because of the plasticizing effect of fatty ester chains of neem oil inside the clay platelets, which increases the flexibility and toughness of the material by increasing the free volume between the molecules. Thus, it provides more ways of energy dissipation by the mobility of exfoliated clay platelets11 and by the segmental motion of the aliphatic ester moieties in their molecular chains.30,31 It has already been shown through XRD study that neem oil increased the spacing (1.17 nm) between the clay layers, which helps to penetrate the hyperbranched epoxy chains into the clay galleries. This helps in the formation of strong interactions between polymer chains and clay layers, which in turn enhances the tensile strength significantly. Elongation at break increases with increase in the amount of NO–OMMT or immobilized neem oil (in the case of PENC2.5). This is due to the increase of plasticization effect of neem oil. However, tensile strength decreases at a higher loading of NO–OMMT (PNC5) and a high amount of immobilized neem oil (PENC2.5). This is because of a decrease in interactions between hyperbranched epoxy and clay platelets at higher amounts of clay loading by the aggregation of some clay platelets, whereas in the case of high amounts of immobilized neem oil, the amount of aliphatic fatty ester chains are increased in the entire system, which provides a more plasticizing effect to the system. The nanocomposites exhibited high scratch hardness, impact resistance and flexibility as the toughness of the hyperbranched epoxy was increased dramatically after the formation of the nanocomposites. However, these differences could not be measured as the values for the nanocomposites reached the highest limit of the instruments for scratch hardness (10 kg) and impact resistance (100 cm); and the lowest limit of the instruments for flexibility evaluation (1 mm bending diameter of mandrel). Nanocomposites absorbed the highest limit of impact energy, which is attributed to the presence of aliphatic fatty esters of neem oil and ether linkages of hyperbranched epoxy, which dissipate the impact energy by the segmental motion in their molecular chains.30,31Antimicrobial activity of the nanocomposites
Neem oil has been used as a biopesticide for a long time. The antibacterial activity of PNC1, PNC2.5 and PNC5 is shown in Fig. 6. From the figure, it can be seen that the nanocomposite exhibited significant antimicrobial activity towards gram positive as well as gram negative bacterial strains. The bacterial zones of inhibition for the nanocomposites were found to be in the range of 16–18 mm in Fig. 7a. However, from Fig. 7b, it can be found that PNC1, PNC2.5 and PNC5 cannot show significant antifungal activity. A slight antifungal activity was observed after the addition of 10 wt% NO–OMMT to the system as shown in Fig. 7b, whereas PENC2.5 exhibited significant antifungal activity towards Candida albicans fungal strains as shown in Fig. 7c. The fungal growth curves for the nanocomposites PNC1, PNC2.5, PNC5 and PENC2.5 are shown in Fig. 7d, in which it can be seen that the nanocomposites inhibit more fungal growth compared to the control. The antifungal activity increases with an increase in the amount of NO–OMMT and immobilized neem oil (PENC2.5). The bacterial growth curves for PNC1, PNC2.5, PNC5 and PENC2.5 are shown in Fig. 8 with gram positive (Bacillus subtilis and Staphylococcus aureus) as well as gram negative (Klebsiella pneumoniae and Pseudomonas aeruginosa) bacterial strains. In the figure, the bacterial growth decreases with an increase in the amount of NO–OMMT and immobilized neem oil (PENC2.5). From this study, it was found that PENC2.5 exhibited the highest antimicrobial activity.Biofilm formation study
A biofilm formation study was performed only for P and PENC2.5 against Candida albicans fungal strain. Fig. 9 showed adherence of more number of fungus on P film compared to PENC2.5. Thus, it is clearly visible that PENC2.5 inhibited more fungal adherence from the surface compared to P, which is due to the presence of neem oil on the OMMT surface of the nanocomposite. The presence of aliphatic fatty ester chains of neem oil in the system also increases the hydrophobicity of the thermoset, which inhibits fungal adherence on the surface.Thermal stability
Thermal stability of the pristine hyperbranched epoxy was increased up to 15–20 °C after the formation of nanocomposites with NO–OMMT as shown in Fig. 10. The initial (5% weight loss) thermal degradation temperature of the pristine hyperbranched epoxy thermoset and its nanocomposites are given in Table 2. The thermal stability of the nanocomposites increases with an increase in the amount of NO–OMMT; however, it decreases with an increase in the amount of immobilized neem oil. When the amount of neem oil increases, the amount of aliphatic fatty ester also increased in the system, which is thermally less stable and thus thermal stability decreases. However, this thermal stability is much higher than the pristine thermoset. The reason for the increase in thermal stability of the pristine thermoset after formation of the nanocomposites is due to the intercalation of clay galleries with hyperbranched epoxy and poly(amido-amine) chains, which restricted the segmental motion of the polymer chains by different physico-chemical interactions.32 In addition, the improvement in the thermostability of the nanocomposite by the incorporation of OMMT is due to the fact that clay is a heat insulator and acts as mass transport barrier to the volatile products generated during decomposition by providing longer paths for them to travel.28 However, the amount of weight residue at 700 °C is found to be haphazard (Fig. 10) and may be due to the volatilization of the modified nanomaterial, which contains thermolabile aliphatic chains.Chemical resistance
The chemical resistance of the pristine thermoset and its nanocomposites was tested in 5% aq. NaOH, 10% aq. HCl, 20% aq. NaCl, 20% aq. EtOH and tap water for 15 days, and the percentage of weight loss of the samples was measured as shown in Table 3. The chemical resistance mainly depends on the cross-linking density and number of chemically stable linkages present in the system. In the case of polymer nanocomposites, it also depends on the interactions between the polymer and the nanomaterials. A small amount of weight loss was observed both in the pristine hyperbranched epoxy thermoset as well as in the nanocomposite films. The percentage of weight loss of nanocomposites in different chemical environments increased at higher amounts of NO–OMMT (PNC5) and immobilized neem oil (PENC2.5). This may be due to the plasticizing effect of the fatty ester of neem oil. This effect creates high free volume to the system, which may help to penetrate the chemicals. These penetrated chemicals degrade the uncured parts of the thermosets, and thus weight loss took place. PNC1, PNC2.5 and PC2.5 showed superior chemical resistance to the pristine system due to the presence of stronger physicochemical interactions like polar–polar, hydrogen bonding, intermolecular, and mutual crosslinking between the matrix and the clay layers by the strong dispersion of clay platelets into the matrix.28| Chemical environment | P | PC2.5 | PNC1 | PNC2.5 | PNC5 | PENC2.5 |
|---|---|---|---|---|---|---|
| Aq. NaOH (5%) | 1.50 | 1.24 | 1.43 | 1.52 | 1.69 | 2.32 |
| Aq. HCl (10%) | 0.92 | 0.87 | 0.82 | 0.90 | 1.02 | 1.92 |
| Aq. NaCl (20%) | 0.32 | 0.22 | 0.24 | 0.33 | 0.45 | 0.97 |
| Aq. EtOH (20%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Water | 0 | 0 | 0 | 0 | 0 | 0 |
Conclusion
The present study demonstrated a facile approach to obtain thermosetting nanocomposites with significant antimicrobial activity, high mechanical properties, good thermal stability and high chemical resistance through the incorporation of neem oil-immobilized organo-modified montmorillonite in hyperbranched epoxy matrix. The investigation also paves a method for addressing the main drawbacks of brittleness and low toughness characteristics of the epoxy thermoset. The intrinsic nature of neem oil as a biopesticide provides antimicrobial activity against different bacterial (both gram positive and gram negative) and fungal strains. Thus, the study opens up a new avenue for polymer nanocomposites to achieve antimicrobial functional material by incorporating neem oil-modified nanomaterial for its potential industrial applications.Acknowledgements
The authors express their gratitude to the NRB for financial assistance through grant no. DNRD/05/4003/NRB/251, dated 29.02.12. SAIF of NEHU, Shillong, is gratefully acknowledged for TEM imaging.Notes and references
- D. Shah, P. Maiti, E. Gunn, D. F. Schmidt, D. D. Jiang, C. A. Batt and E. P. Giannelis, Adv. Mater., 2004, 16, 1173 CrossRef CAS.
- I. Park, H. Peng, D. W. Gidley, S. Xue and T. J. Pinnavaia, Chem. Mater., 2006, 18, 650 CrossRef CAS.
- B. Chen, J. R. G. Evans, H. C. Greenwell, P. Boulet, P. V. Coveney, A. A. Bowden and A. Whiting, Chem. Soc. Rev., 2008, 37, 568 RSC.
- K. Wang, L. Chen, J. Wu, M. L. Toh, C. He and A. F. Yee, Macromolecules, 2005, 38, 788 CrossRef CAS.
- J. H. Park and S. C. Jana, Macromolecules, 2003, 36, 2758 CrossRef CAS.
- S. Barua, N. Dutta, S. Karmakar, P. Chattopadhyay, L. Aidew, A. K. Buragohain and N. Karak, Biomed. Mater., 2014, 9, 025006 CrossRef PubMed.
- N. Banik, M. Iman, A. Hussain, A. Ramteke, R. Baruah and T. K. Maji, New J. Chem., 2013, 37, 3981 RSC.
- T. Pongprayoon, R. Nuangchamnong and N. Yanumet, Appl. Clay Sci., 2013, 86, 179 CrossRef CAS PubMed.
- S. Barua, P. Chattopadhyay, L. Aidew, A. K. Buragohain and N. Karak, Polym. Int., 2014 DOI:10.1002/pi.4790.
- B. Bagchi, S. Kar, S. K. Dey, S. Bhandary, D. Roy, T. K. Mukhopadhyay, S. Das and P. Nandy, Colloids Surf., B, 2013, 108, 358 CrossRef CAS PubMed.
- B. De and N. Karak, J. Appl. Polym. Sci., 2014, 131, 40327 CrossRef.
- A. Hartwig, D. Putz, B. Schartel, M. Bartholmai and M. Wendschuh-Josties, Macromol. Chem. Phys., 2003, 204, 2247 CrossRef CAS.
- W. S. Wang, H. S. Chen, Y. W. Wu, T. Y. Tsai and Y. W. Chen-Yang, Polymer, 2008, 49, 4826 CrossRef CAS PubMed.
- B. De and N. Karak, J. Mater. Chem. A, 2013, 1, 348 CAS.
- B. De, K. Gupta, M. Mandal and N. Karak, ACS Sustainable Chem. Eng., 2014, 2, 445 CrossRef CAS.
- D. Zhang and D. Jia, J. Appl. Polym. Sci., 2006, 101, 2504 CrossRef CAS.
- Y. Wu, N. Zhou, W. Li, H. Gu, Y. Fan and J. Yuan, Mater. Sci. Eng., C, 2013, 33, 752 CrossRef PubMed.
- M. S. Usman, M. E. E. Zowalaty, K. Shameli, N. Zainuddin, M. Salama and N. A. Ibrahim, Int. J. Nanomed., 2013, 8, 4467 Search PubMed.
- X. Liang, M. Sun, L. Li, R. Qiao, K. Chen, Q. Xiao and F. Xu, Dalton Trans., 2012, 41, 2804 RSC.
- M. SaiRam, G. Ilavazhagan, S. K. Sharma, S. A. Dhanraj, B. Suresh, M. M. Parida, A. M. Jana, K. Devendra and W. Selvamurthy, J. Ethnopharmacol., 2000, 71, 377 CrossRef CAS.
- N. A. Ibrahima, B. M. Eida and E. R. El-Zairy, Carbohydr. Polym., 2011, 86, 1313 CrossRef PubMed.
- S. Ismadji, A. Kurniawan, Y. H. Ju, F. E. Soetaredjo, A. Ayucitra and L. K. Ong, Fluid Phase Equilib., 2012, 336, 9 CrossRef CAS PubMed.
- A. U. Anya, N. N. Chioma and O. Obinna, J. Basic Appl. Chem., 2012, 2, 21 Search PubMed.
- B. De, B. Voit and N. Karak, ACS Appl. Mater. Interfaces, 2013, 5, 10027 CAS.
- P. Radhika, B. S. Sastry and B. M. Harica, Res. J. Biotechnol., 2008, 3, 62 Search PubMed.
- B. Roy, P. Bharali, B. K. Konwar and N. Karak, Bioresour. Technol., 2013, 127, 175 CrossRef CAS PubMed.
- D. Djordjevic, M. Wiedmann and L. A. McLandsborough, Appl. Environ. Microbiol., 2002, 68, 2950 CrossRef CAS.
- B. Roy, P. Bharali, B. K. Konwar and N. Karak, Int. J. Mater. Res., 2014, 105, 296 CrossRef CAS.
- R. Wang, T. Schuman, R. R. Vuppalapati and K. Chandrashekhara, Green Chem., 2014, 16, 1871 RSC.
- S. Pathan and S. Ahmad, ACS Sustainable Chem. Eng., 2013, 1, 1246 CrossRef CAS.
- S. Pathan and S. Ahmad, J. Mater. Chem. A, 2013, 1, 14227 CAS.
- S. B. Khan, K. Akhtar, M. M. Rahman, A. M. Asiri, J. Seo, K. A. Alamry and H. Han, New J. Chem., 2012, 36, 2368 RSC.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |