Large-scale synthesis of PEGylated lutetium hydroxycarbonates as nanoparticulate contrast agents for X-ray CT imaging†
Zhaogui
Ba‡
ab,
Yumin
Zhang‡
b,
Junpei
Wei
c,
Jiwu
Han
b,
Zhenqiang
Wang
b and
Guangrui
Shao
*a
aDepartment of Radiology, The Second Hospital of Shandong University, Shandong University, Jinan, Shandong 250033, P. R. China. E-mail: shaoguangrui@yeah.net
bDepartment of Radiology, Laigang Hospital Affiliated to Taishan Medical University, Laiwu, Shandong 271126, P. R. China
cDepartment of Radiology, People's Hospital of Xintai City, Xintai, Shandong 271200, P. R. China
First published on 13th October 2014
Abstract
Nanoparticulate contrast agents have drawn considerable attention and interest because of their potential application in medical diagnosis and prognosis. In the present study, we designed and constructed high-performance nanoparticulate contrast agents based on PEGylated lutetium hydroxycarbonate nanoparticles (PEG-LuNPs) for X-ray computed tomography imaging, which were synthesized via a green and large-scale route. Under the daily clinical voltage, our PEG-LuNPs provided much more enhanced contrast than the routinely used iodine-based molecules. More importantly, PEG-LuNPs could act as liver-targeted contrast agents for the further detection of hepatic metastases. Both in vitro and in vivo toxicity studies indicated that these nanoparticles possess extremely high biocompatibility, revealing their overall safety. Based on these results, PEG-LuNPs with intrinsic physicochemical properties and excellent imaging capability demonstrated a useful nanoplatform for biomedical applications.
Introduction
Due to its cost effectiveness, deep tissue penetration, and high resolution, X-ray computed tomography (CT) has been regarded as one of the most powerful diagnostic imaging techniques along with the rapid development of modern medicine.1–5 Small iodinated molecules are routinely used as CT contrast agents in the clinical setting. However, these contrast agents can effectively absorb X-rays but have limitations due to rapid renal clearance, vascular permeation, and low specificity.6–10 Moreover, synthesis and purification of these iodine-based small molecules usually depend on multi-step methodologies. Thereby, the research focused on the development of novel CT contrast agents is essential.Smart nano-engineered materials have moved into the spotlight as various high-resolution contrast agents owing to their excellent physicochemical properties.11–15 In particular, heavy metal-based nanoparticles can strongly absorb X-ray radiations and enhance imaging contrast by several folds even at low X-ray doses during the CT imaging.16–20 Compared with small iodinated molecules, these nanoparticulate contrast agents possess high contrast densities and long blood circulation periods, showing promise in in vivo targeted imaging and angiography.21–23 In addition, these agents can display prominent superiority in imaging efficacy with respect to small iodinated molecules under clinical operating voltages ranging from 80 kVp to 140 kVp. More importantly, these iodine-free nanoparticles cannot result in an iodine-induced hypersensitivity reaction in the clinic. Because a large amount of CT contrast agents is highly required during the imaging process, the following criteria must be cited with intense interest: (1) a suitable X-ray attenuation coefficient under clinical operation; (2) high biocompatibility and low systemic toxicity; (3) cost effectiveness and facile synthesis. Among all currently available heavy metal-based nanomaterials, lanthanide-based particles hold great promise as CT contrast agents and have been used in CT imaging owing to their suitable K-edge energy located within the high-energy region of the X-ray spectrum and high abundance on earth.24–28 For example, lanthanide-doped NaYbF4 nanoprobes were designed as the first Yb-based CT contrast agents for CT imaging.29–31 Nanoparticles based on lanthanide oxides were prepared for multimodal imaging with extremely low systemic toxicity.32–34 Pro-drug-conjugated upconversion nanomaterials were used for multimodal imaging and NIR light-triggered anti-cancer treatment.35 Although promising, there are still many intractable problems that hinder the development of this field, such as time-consuming and multi-step synthesis routes to construct nanoparticulate contrast agents. More importantly, it is highly in demand to obtain inexpensive and low-toxicity nano-CT contrast agents with facile and large-scale routes.
A previous study demonstrated that a urea-based homogeneous precipitation method could act as a main route to prepare colloidal single-lanthanide hydroxycarbonates.36 Very recently, Gd-doped Yb(OH)CO3 nanoparticles have been constructed and applied as dual-modal contrast agents for X-ray computed tomography/magnetic resonance (CT/MR) imaging.37 However, this direct synthesis without any surface modification can decrease the dissolvability of nanoparticulate contrast agents in physiological solutions and hence cause serious aggregation in the animal body, indicating the inapplicability of these nanoagents in vivo. Inspired by above studies, a modified strategy based on the urea-assisted homogeneous precipitation route was applied to prepare PEGylated Lu(OH)CO3 nanoparticles (PEG-LuNPs) by doping polyethylene glycol (PEG) molecules in the synthesis process. With extremely low cytotoxicity and hemolytic activity, these contrast agents exhibited excellent efficiency in CT imaging with respect to small iodinated molecules. Moreover, PEG-LuNPs could be effectively accumulated into the liver of experimental animals and act as liver-targeted contrast agents, showing promise for the further detection of hepatic metastases. Long-term toxicity studies including body weight analysis, histology assay, and blood biochemistry assay were also carried out after a single-dose intravenous injection in a mouse model, revealing the overall safety of our well-prepared contrast agents.
Experimental section
Materials
Lutetium chloride hexahydrate (LuCl3·6H2O), chloral hydrate, polyethylene glycol (PEG-2000) and urea were obtained from Aladdin Reagent. Iobitridol was purchased from Guerbet. Other reagents and solvents were acquired from Beijing Chemicals.Preparation of PEGylated Lu(OH)CO3 nanoparticles
PEGylated Lu(OH)CO3 nanoparticles were prepared via a one-pot urea-based homogeneous precipitation process. Typically, LuCl3·6H2O (6.0 mmol), PEG-2000 (2.0 g), and urea (200 mmol) were dissolved in deionized water (400 mL). After magnetic stirring at room temperature for 2 h, the resultant homogeneous solution was reacted at 90 °C for another 3 h. The product was collected after washing with deionized water and ethanol in sequence, and dried under vacuum at 60 °C overnight for further use.Cell cultures
Hela cells were supplied by American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (DMEM) containing streptomycin (100 U mL−1), 10% fetal bovine serum (FBS) and penicillin (100 U mL−1), in a humidified incubator at 37 °C and 5% CO2. The cells were harvested by the use of trypsin and were placed back into a fresh complete medium before plating.In vitro cytotoxicity studies
To quantify the cytotoxicity of PEG-LuNPs, MTT assays were carried out. Hela cells were cultured in 96-well plates with a density of 5 × 103 per well for 12 h to allow the cells to attach. Serial dilutions of different agent formulations were added to the culture medium. One day later, the medium containing PEG-LuNPs were removed, and cell samples were treated with MTT for another 4 h. Dimethyl sulfoxide (DMSO) was used to dissolve the formazan crystals. Six replicates were done in each group and the percent viability was normalized to the cell viability without treatment.Observation of cellular modality
Hela cells with a density of 2 × 104 were plated in a 12-well plate for 6 h to allow the cells to attach. PEG-LuNPs (0.4 mg mL−1) were added to the cell culture medium. After 24 h of incubation, cells were washed several times with 0.9% NaCl to remove the remaining nanoparticles, stained with trypan blue, and then observed under an optical microscope.In vitro hemolysis assay
Hemolysis assay experiments were performed to evaluate the in vitro biocompatibility. Blood samples (1 mL) were added into 0.9% NaCl (3 mL), and then red blood cells (RBCs) were isolated from the serum by centrifugation. After being washed several times with 0.9% NaCl, the purified blood was diluted to 1/10 of its volume with 0.9% NaCl. Diluted RBC suspension (0.2 mL) was then mixed with (a) 0.9% NaCl (0.8 mL) as a negative control, (b) D. I. water (0.8 mL) as a positive control, and (c) suspensions (0.8 mL) of PEG-LuNPs with concentrations ranging from 0 to 1 mg mL−1. All the mixtures were vortexed and kept at room temperature for 3 h. Finally, the mixtures were centrifuged, and the absorbance of supernatants at 541 nm was determined using a UV-vis-NIR spectrophotometer.Animal administration
Wister rats, Kunming mice, and C57BL/6 mice were obtained from the Laboratory Animal Center of Shandong University (Jinan, China). There handling and care procedures were under the jurisdiction and guidelines of the Regional Ethics Committee for Animal Experiments.Establishment of a tumour-bearing model
A subcutaneous transplantable mouse model of lung cancer was prepared by injecting Lewis lung carcinoma cells (1 × 106) in 0.9% NaCl (0.1 mL) at the right axillary fossa of C57BL/6 mice.CT imaging
To assess CT contrast efficacy, PEG-LuNPs and iobitridol were dispersed in NaCl containing 1% agarose with different Lu and I concentrations. The Eppendorf tubes were scanned in a Philips CT imaging system. For in vivo imaging, rats were firstly anesthetized by intraperitoneally injecting chloral hydrate (10 wt%). PEG-LuNPs in 0.9% NaCl (100 mg kg−1) were injected intravenously. CT images were then acquired using a Philips Medical System. The clinical operation voltage was 120 kVp.Change in body weight
Kunming mice were separated into two cages (n = 6). The mice in the test group were injected intravenously with PEG-LuNPs (200 mg kg−1). In addition, mice injected with 0.9% NaCl were selected as the control group. We recorded the change in body weight for a month.Assay of histology and blood biochemistry
All the mice in both groups were sacrificed a month later. Main exposed organs including the heart, liver, spleen, lungs, and kidneys were collected. These organs were then fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned (4 μm thick), and stained with hematoxylin and eosin (H&E). The histological sections were observed under an optical microscope. Moreover, blood from above groups was collected to carry out blood biochemical assay.Results and discussion
Synthesis and characterisation of PEGylated Lu(OH)CO3 nanoparticles
Fig. 1 clearly illustrates our design and synthesis of PEGylated Lu(OH)CO3 nanoparticles, as well as their application as X-ray CT contrast agents. Typical construction of PEG-LuNPs was performed via a modified urea-based homogeneous precipitation method by doping PEG molecules in the synthesis process. Due to self-decomposition of urea into OH− and CO32− at high temperature, this classical method has been considered as a general route for the preparation of lanthanide hydroxylcarbonate. By doping PEG molecules, our nanoparticulate contrast agents could be dispersed well in various physiological solutions. The SEM image and the TEM image shown in Fig. 2A and B revealed that our PEG-LuNPs presented a non-aggregated and spherical nature with a smooth surface. Particle-size distribution obtained from the TEM result shown in Fig. 3C indicated that PEG-LuNPs had a mean diameter of 115 nm. To test the accurate hydrodynamic size of PEG-LuNPs, dynamic light scattering (DLS) was further carried out, indicating that the well-prepared nanoparticles had an average diameter of 135 nm with a standard deviation of ±21.4 nm. Energy-dispersive spectroscopy (EDS) analysis via the point scan mode revealed the presence of Lu, C, and O elements in PEG-LuNPs (Fig. 2D). FT-IR spectra revealed the characteristic absorption bands of VasO–C–O (1530 and 1406 cm−1), π-CO32− (844 cm−1), and δ-CO32− (762 and 696 cm−1), which were in accordance with those of the carbonate group (Fig. 2E).37 Moreover, the bands around 3000 cm−1 in the spectrum were assigned to C–H stretching vibration, indicating the successful modification of PEG molecules on the surface of nanoparticles.38 Considering that a large amount of CT contrast agents are highly required in the clinic, large-scale production of nanoagents must be achieved via a facile process. As illustrated in Fig. 2F, the large-scale production of PEG-LuNPs could be easily obtained via increasing the amount of reagents and the product exhibited a white color. Compared with previous methods, our present method is free of organic reagents and shows a facile fabrication route. | ||
| Fig. 1 Schematic illustration for the synthesis of PEG-LuNPs and their application as X-ray CT contrast agents. | ||
In vitro toxicity
From the viewpoint of diagnosis and prognosis in clinical settings, contrast agents must be non-toxic and biocompatible. Although nanoparticles based on lanthanide hydroxylcarbonates are well known to be less cytotoxic, we also carried out a methyl thiazolyl tetrazolium (MTT) assay to test the cytotoxicity of PEG-LuNPs, which must be established before small-animal experiments. After one-day exposure to PEG-LuNPs with various concentrations, results of viability of Hela cells exhibited that more than 90% cells survived (Fig. 3A). In the presence of our nanoparticulate contrast agents at a concentration of 0.4 mg mL−1, microscopy images of Hela cells illustrated no obvious difference in the cell morphology for the cells treated with PEG-LuNPs compared to the control group (Fig. 3B). Hemolysis assay was additionally performed to evaluate the blood compatibility.39,40 UV-vis spectra and photographic images further demonstrated that PEG-LuNPs caused no hemolysis of RBCs even upon the maximal experimental concentration (1 mg mL−1). On the basis of these results, it could be inferred that PEG-LuNPs were highly compatible towards both Hela cells and RBCs, thus implying that PEG-LuNPs could serve as safe CT contrast agents for in vivo CT imaging.CT imaging
In preliminary in vivo animal experiments, we first examined the contrast efficiency of PEG-LuNPs relative to iobitridol (a popular iodine-based X-ray CT contrast agent in the clinic). Fig. 4A and B display that both contrast agents exhibited signal enhancement with an increase of the contrast agent concentration, and samples containing higher concentrations of contrast agents appeared brighter on CT images. A good linear correlation between the Hounsfield unit (HU) values and the concentration of Lu or I was observed at the same time. Notably, the obtained HU values of PEG-LuNPs were significantly enhanced compared to iobitridol at equivalent concentration of each agent. These results implied that our PEG-LuNPs might be applied at a reduced dosage, thus lowering the possible adverse side effects. Having established high biocompatibility and high contrast efficiency, in vivo CT imaging studies with PEG-LuNPs were next performed on a clinical CT system. Owing to the high atomic number and electron density of lanthanon elements, PEG-LuNPs could result in enhanced positive contrast around the injection sites compared with other soft tissues. For primary in vivo CT imaging, PEG-LuNPs were intratumourally injected into a tumour-bearing mouse. Fig. 4C reveals an obvious enhancement of the CT signal around the tumour site after injection. Encouraged by our above results, we further evaluated the whole body CT imaging by intravenous injection of PEG-LuNPs and assessed the biodistribution of contrast agents tracked by the clinical CT system. The rat was anesthetized first and injected intravenously with PEG-LuNP dispersion in 0.9% NaCl solution. Fig. 4D presents the coronal view and 3D-renderings of CT images of the rat. Once the solution containing PEG-LuNPs was injected, a clear signal enhancement of the liver was observed at an early time (20 min). Remarkably, the gradual signal enhancement of liver and spleen continued for over 60 min. More careful observations via 3D-renderings of CT images also provided evident signal enhancement of liver vessels. According to the above results, our PEG-LuNPs could act as high-performance liver-targeted CT contrast agents. Compared with small iodinated molecules, PEG-LuNPs decorated with an anti-biofouling polymer could prolong the blood circulation period and overcome the limitation in targeted imaging and angiography. Different from normal tissues, tumour tissues can uptake more contrast agents during the imaging process owing to the intrinsic enhanced permeation and retention effect (EPR).7,21,27 More importantly, our PEG-LuNPs accumulated in liver could serve as useful CT contrast agents for the detection of hepatic metastases.Investigation of long-term toxicity
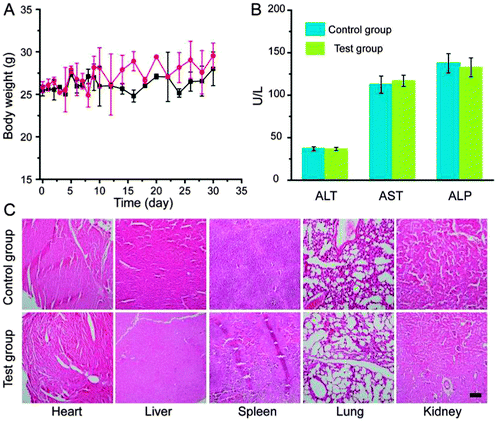
Last but not least, we tested the long-term toxicity of PEG-LuNPs. After intravenous injection of a single dose of PEG-LuNPs, all the mice remained healthy over a one-month period. No abnormalities in eating, drinking, activity, exploratory behaviour, or neurological status were noticed at the same time.41–46 As shown in Fig. 5A, body weight of the test group increased slightly in a pattern similar to that of the control group. One month after injection, several important hepatic indicators such as alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) were selected to carry out blood biochemical assays. All the measured parameters fell within the normal ranges and revealed no sign of liver injury (Fig. 5B). Mice in both groups were then sacrificed for careful necropsy. To determine whether these nanoparticles caused any tissue damages or any other toxic effect on mice, major organs including the heart, liver, spleen, lungs, and kidneys were sliced and stained by hematoxylin and eosin (H&E) for histological assessments. Fig. 5C reveals that no noticeable tissue damages or any other toxic effect on organs occurred. However, many careful studies are still needed to examine the potential toxicity of PEG-LuNPs with a much longer term, which is significant for the further application of this type of nanoparticulate contrast agents in biomedicine.Conclusions
In summary, we reported here a facile strategy to synthesize monodispersed X-ray CT contrast agents based on PEGylated lutetium hydroxycarbonate nanoparticles via a modified urea-based homogeneous precipitation method by doping PEG molecules. Detailed evaluation of cytotoxicity and hemolysis demonstrated the excellent biocompatibility and extremely low cytotoxicity of our PEGylated Lu(OH)CO3 nanoparticles. Compared with routinely used iobitridol in clinic, our nanoparticulate contrast agents could provide much obvious enhancement under clinical voltages. More importantly, the liver-targeted CT contrast agents presented more potential in further detection of hepatic metastases due to their efficient accumulation in the liver after intravenous injection. In addition, long-term toxicity studies indicated that our well-prepared nanoparticulate CT contrast agents possess overall safety and show promise for further biomedical usages.References
- T. Skotland, Contrast Media Mol. Imaging, 2012, 7, 1 CrossRef CAS PubMed.
- S. Yu and A. Watson, Chem. Rev., 1999, 99, 2353 CrossRef CAS PubMed.
- J. Kim, Y. Piao and T. Hyeon, Chem. Soc. Rev., 2009, 38, 372 RSC.
- G. Maltzahn, J. Park, A. Agrawal, N. Bandaru, S. Das, M. Sailor and S. Bhatia, Cancer Res., 2009, 69, 3892 CrossRef PubMed.
- W. Cai and X. Chen, Small, 2007, 3, 1840 CrossRef CAS PubMed.
- A. Jakhmola, N. Anton and T. Vandamme, Adv. Healthcare Mater., 2012, 1, 413 CrossRef CAS PubMed.
- H. Wang, L. Zheng, C. Peng, R. Guo, M. Shen, X. Shi and G. Zhang, Biomaterials, 2011, 32, 2979 CrossRef CAS PubMed.
- M. Shilo, T. Reuveni, M. Motiei and R. Popovtzer, Nanomedicine, 2012, 7, 257 CrossRef CAS PubMed.
- S. Zeng, M. Tsang, C. Chan, K. Wong and J. Hao, Biomaterials, 2012, 33, 9232 CrossRef CAS PubMed.
- J. Zhou, M. Yu, Y. Sun, X. Zhang, X. Zhu, Z. Wu, D. Wu and F. Li, Biomaterials, 2011, 3, 1148 CrossRef PubMed.
- K. Park, S. Lee, E. Kang, K. Kim, K. Choi and I. Kwon, Adv. Funct. Mater., 2009, 19, 1553 CrossRef CAS.
- L. Cheng, K. Yang, S. Zhang, M. Shao, S. Lee and Z. Liu, Nano Res., 2010, 3, 722 CrossRef CAS PubMed.
- G. Tian, Z. Gu, L. Zhou, W. Yin, X. Liu, L. Yan, S. Jin, W. Ren, G. Xing, S. Li and Y. Zhao, Adv. Mater., 2012, 24, 1226 CrossRef CAS PubMed.
- Y. Wang, H. Wang, D. Liu, S. Song, X. Wang and H. Zhang, Biomaterials, 2013, 34, 7715 CrossRef CAS PubMed.
- M. Oh, N. Lee, H. Kim, S. Park, Y. Piao, J. Lee, S. Jun, W. Moon, S. Choi and T. Hyeon, J. Am. Chem. Soc., 2011, 133, 5508 CrossRef CAS PubMed.
- C. Alric, J. Taleb, G. Duc, C. Mandon, C. Billotey, A. Meur-Herland, T. Brochard, F. Vocanson, M. Janier, P. Perriat, S. Roux and O. Tillement, J. Am. Chem. Soc., 2008, 130, 5908 CrossRef CAS PubMed.
- Q. Yin, F. Yap, L. Yin, L. Ma, Q. Zhou, L. Dobrucki, T. Fan, R. Gaba and J. Cheng, J. Am. Chem. Soc., 2013, 135, 13620 CrossRef CAS PubMed.
- A. Xia, Y. Gao, J. Zhou, C. Li, T. Yang, D. Wu, L. Wu and F. Li, Biomaterials, 2011, 32, 7200 CrossRef CAS PubMed.
- D. Pan, C. Schirra, A. Senpan, A. Schmieder, A. Stacy, E. Roessl, A. Thran, S. Wichline, R. Proska and G. Lanza, ACS Nano, 2012, 6, 3364 CrossRef CAS PubMed.
- Z. Liu, F. Pu, J. Liu, L. Jiang, Q. Yuan, Z. Li, J. Ren and X. Qu, Nanoscale, 2013, 5, 4252 RSC.
- X. Zhu, J. Zhou, M. Chen, M. Shi, W. Feng and F. Li, Biomaterials, 2012, 33, 4618 CrossRef CAS PubMed.
- K. deKrafft, W. Boyle, L. Burk, O. Zhou and W. Lin, J. Mater. Chem., 2012, 22, 18139 RSC.
- H. Xing, W. Bu, Q. Ren, X. Zheng, M. Li, S. Zhang, H. Qu, Z. Wang, Y. Hua, K. Zhao, L. Zhou, W. Peng and J. Shi, Biomaterials, 2012, 33, 5384 CrossRef CAS PubMed.
- D. Pan, C. Schirra, S. Wickline and G. Lanza, Contrast Media Mol. Imaging, 2014, 9, 13 CrossRef CAS PubMed.
- Y. Wu, Y. Sun, X. Zhu, Q. Liu, T. Cao, J. Peng, Y. Yang, W. Feng and F. Li, Biomaterials, 2014, 35, 4699 CrossRef CAS PubMed.
- M. He, P. Huang, C. Zhang, H. Hu, C. Bao, G. Gao, R. He and D. Cui, Adv. Funct. Mater., 2011, 21, 4470 CrossRef CAS.
- Q. Xiao, X. Zheng, W. Bu, W. Ge, S. Zhang, F. Chen, H. Xing, Q. Ren, W. Fan, K. Zhao, Y. Hua and J. Shi, J. Am. Chem. Soc., 2013, 135, 13041 CrossRef CAS PubMed.
- D. Yang, Y. Dai, J. Liu, Y. Zhou, Y. Chen, C. Li, P. Ma and J. Lin, Biomaterials, 2014, 35, 2011 CrossRef CAS PubMed.
- Y. Liu, K. Ai, J. Liu, Q. Yuan, Y. He and L. Lu, Angew. Chem., Int. Ed., 2012, 51, 1437 CrossRef CAS PubMed.
- Y. Liu, K. Ai, J. Liu, Q. Yuan, Y. He and L. Lu, Adv. Healthcare Mater., 2012, 1, 461 CrossRef CAS PubMed.
- Y. Liu, K. Ai and L. Lu, Acc. Chem. Res., 2012, 45, 1817 CrossRef CAS PubMed.
- Z. Liu, Z. Li, J. Liu, S. Gu, Q. Yuan, J. Ren and X. Qu, Biomaterials, 2012, 33, 6748 CrossRef CAS PubMed.
- Z. Liu, F. Pu, S. Huang, Q. Yuan, J. Ren and X. Qu, Biomaterials, 2013, 34, 1712 CrossRef CAS PubMed.
- Z. Liu, K. Dong, J. Liu, X. Han, J. Ren and X. Qu, Small, 2014, 10, 2429 CrossRef CAS PubMed.
- Y. Dai, H. Xiao, J. Liu, Q. Yuan, P. Ma, D. Yang, C. Li, Z. Cheng, Z. Hou, P. Yang and J. Lin, J. Am. Chem. Soc., 2013, 135, 18920 CrossRef CAS PubMed.
- E. Matijevic and W. Hsu, J. Colloid Interface Sci., 1987, 118, 506 CrossRef CAS.
- Y. Jin, J. Liu, Q. Zheng, J. Xu, B. Sharma, G. He, M. Yin, L. Zhang, Y. Song, T. Li, Q. Yuan, Y. Sun and H. Yang, New J. Chem., 2013, 37, 3024 RSC.
- Z. Yue, W. Wei, Z. You, Q. Yang, H. Yue, Z. Su and G. Ma, Adv. Funct. Mater., 2011, 21, 3446 CrossRef CAS.
- I. Slowing, C. Wu, J. Vivero-Escoto and V. Lin, Small, 2009, 5, 4834 CrossRef PubMed.
- Y. Lin and C. Haynes, J. Am. Chem. Soc., 2010, 132, 4834 CrossRef CAS PubMed.
- L. Cheng, K. Yang, M. Shao, X. Lu and Z. Liu, Nanomedicine, 2011, 6, 1327 CrossRef CAS PubMed.
- Y. Liu, K. Ai, J. Liu, M. Deng, Y. He and L. Lu, Adv. Mater., 2013, 25, 1353 CrossRef CAS PubMed.
- C. Peng, L. Zheng, Q. Chen, M. Shen, R. Guo, H. Wang, X. Cao, G. Zhang and X. Shi, Biomaterials, 2012, 33, 1107 CrossRef CAS PubMed.
- R. Guo, H. Wang, C. Peng, M. Shen, L. Zheng, G. Zhang and X. Shi, J. Mater. Chem., 2011, 21, 5120 RSC.
- Y. Fang, C. Peng, R. Guo, L. Zheng, J. Qin, B. Zhou, M. Shen, X. Lu, G. Zhang and X. Shi, Analyst, 2013, 138, 3172 RSC.
- H. Liu, H. Wang, Y. Xu, M. Shen, J. Zhao, G. Zhang and X. Shi, Nanoscale, 2014, 6, 4521 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nj01524j |
| ‡ Z. G. Ba and Y. M. Zhang should be regarded as Joint First Authors. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |