Hydrogen-bonded chiral molecular switches: photo- and thermally-reversible switchable full range color in the self-organized helical superstructure
Ouyu
Jin
,
Dengwei
Fu
,
Yixiu
Ge
,
Jie
Wei
and
Jinbao
Guo
*
College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: guojb@mail.buct.edu.cn
First published on 9th October 2014
Abstract
In this study, new kinds of hydrogen-bonded (H-bonded) chiral molecular switches (CMS) composed of azobenzene moieties as photo-responsive parts and H-bonded complexes as thermo-responsive parts were developed. Photo- and thermally-reversible switching behavior of cholesteric liquid crystals (Ch-LCs) based on the H-bonded CMS was investigated in detail. The results demonstrate that H-bonded CMS undergo a trans–cis photoisomerization under UV light, which results in the decrease of helical twisting power (HTP). Meanwhile, the modulation of the intermolecular forces between proton donors and acceptors with temperature has a positive influence on the change in HTP. Reversible blue, green, and red reflections of the self-organized helical superstructure could be achieved under UV/Vis light as well as with temperature change. According to the geometry optimization based on Gaussian 03 calculations at the B3LYP/6-31G(d) level, the molecular aspect ratio could be considered to be one of the important factors influencing the HTP of H-bonded CMS. This dynamic tuning of the self-organized helical superstructure based on dual and selective molecular mechanism opens up a way to achieve new kinds of LC photonic materials.
Introduction
Cholesteric liquid crystals (Ch-LCs), which are characterized by their self-organized helical superstructure, have attracted tremendous attention because of their unique optical properties such as selective reflectivity.1 The pitch refers to the distance over which the LC molecules undergo a full 360° twist and the wavelength of maximum reflection can be described as below: λmax = n × P, where λmax is the spectral position of the reflection, n is the average refractive index and P is the pitch.2 As shown in the equation, the pitch, which is sensitive to external stimuli, plays a very important role in tuning the selective reflection band (SRB) of Ch-LCs. It is widely acknowledged that when achiral nematic liquid crystals (N-LCs) are doped with chiral compounds, they will be transformed into Ch-LCs.3 Since the pitch length of Ch-LCs is sensitive to external factors including heat,4 light irradiation,5 and force,6 stimuli-responsive Ch-LCs are expected, which have been a focus of extensive research interest in the past few decades.7Due to the advantages of the ease of addressability, fast response time and potential for remote control,8 a lot of investigations have been carried out on photoinduced optical responses of Ch-LCs.9 A widely studied class of photoactive molecular switches is photochromic compounds including azobenzenes,10 dithienylethenes,11,12etc. Azobenzene and its derivatives, which are most commonly used dopants, undergo trans–cis and cis–trans isomerization upon UV/Vis irradiation while their cis and trans isomers are bent and rod-like in shape respectively. Studies have proved that the conformation difference in the azobenzene compound induced by light irradiation contributes a lot to affect the helical twisting power (HTP) of chiral dopants so that we can obtain photoresponsive Ch-LCs based on such molecular switches. Among them, Li et al. reported reversible photo-responsive Ch-LCs containing an azobenzene moiety with a short flexible carbonyldioxy spacer and a polar carbonyl unit in a terminal chain and the reversible photoresponsive properties were well demonstrated by UV irradiation. Furthermore, Li et al. also reported a light driven nanoscale chiral molecular switch capable of phototuning the reflection color across the full visible spectrum due to the photoisomerization of binaphthyl azobenzene groups and the HTP of the molecular switch was 304 μm−1 (in the initial state) and 89 μm−1 (in the photostationary state).13
Moreover, hydrogen bonding (H-bond), a typical way of assembling molecules and achieving supramolecular structures, has been an attractive way to construct stimuli-responsive materials.14 For example, owing to the interaction of H-bond donors and acceptors, H-bonded chiral monomers can be used as molecular triggers to obtain Ch-LC polymer network materials that change the reflection color upon a change in temperature as well as the change in pH.15,16 Recently, optical sensors based on H-bonded Ch-LC networks are nicely illustrated by Herzer et al.17 Optical sensors made from them can respond to temperature and humidity by changing their reflection color.
In this work, we developed a facile method to obtain dual responsive Ch-LCs by fabricating new kinds of H-bonded chiral molecular switches (CMS), in which a binaphthyl azobenzene molecule is used as the proton acceptor, and four chiral and achiral acids are proton donors. Here we note that the complicated synthesis of CMS was simplified due to the easy H-bonded assembly process. Based on the H-bonded CMS, the photo- and thermo-responsive behavior of Ch-LCs was investigated in detail. Herein, we focus on the influence of the molecular structure of the proton donors on the HTP of H-bonded CMS and the corresponding switching effects. Additionally, we utilize the molecular simulation to estimate the relationship between the molecular structure of H-bonded CMS and the HTP, the change in HTP and the switching mechanism. The main practical objective of this work is to develop a relatively simple and effective approach to fabricate new kinds of responsive LC photonic materials.
Experimental section
Materials
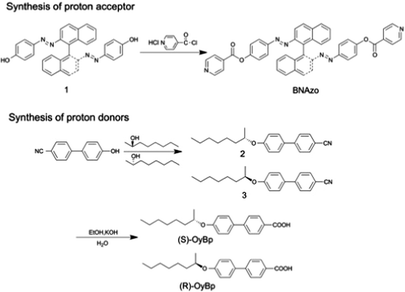
In this study, SLC1717 (isotropic temperature, Ti = 92 °C; 20 °C, 589 nm, Δn = 0.201, Slichem Liquid Crystal Material Co., Ltd), (S)-(+)-2-octanol, and (R)-(−)-2-octanol (Beijing Lyra Material-Tech Co., Ltd) were used. 4-(Decyloxy)benzoic acid (DBA) and (S)-(+)-2-methylbutyric acid (MBA) were purchased from Sigma-Aldrich. Isonicotinoylchloride hydrochloride was synthesized as described in our previous paper.15 The proton acceptor, 4,4′-((S)-[1,1′-binaphthalene]-2,2′-diyl)bis(diazene-2,1-diyl)diphenol isonicotinate (BNAzo) and the proton donors, (S)-4′-(octan-2-yloxy)-[1,1′-biphenyl]-4-carboxylic acid ((S)-OyBp) and (R)-4′-(octan-2-yloxy)-[1,1′-biphenyl]-4-carboxylic acid ((R)-OyBp), were synthesized following the procedures as shown in Scheme 1.Synthesis of H-bonded proton acceptors
4,4′-((S)-[1,1′-Binaphthalene]-2,2′-diyl)bis(diazene-2,1-diyl)diphenol. Compound 1 was synthesized according to the procedure given in previous work.9i,18
1H NMR (400 MHz, DMSO): δ = 10.18 (s, 2H, Ar-OH), 8.18 (d, J = 8.9 Hz, 2H, Ar-H), 8.10 (m, 4H, Ar-H), 7.57 (t, J = 7.5 Hz, 2H, Ar-H), 7.35 (t, J = 8.1 Hz, 2H, Ar-H), 7.28 (d, J = 8.35 Hz, 2H, Ar-H), 7.18 (d, J = 8.73 Hz, 4H, Ar-H), 6.70 (d, J = 8.73 Hz, 4H, Ar-H).
((S)-[1,1′-Binaphthalene]-2,2′-diyl)bis(diazene-2,1-diyl)diphenol isonicotinate (H-bonded proton acceptor). To a stirred suspension of isonicotinoyl chloride hydrochloride (0.07 g, 0.4 mmol) in THF (10 ml), triethylamine (0.45 ml) was added slowly. A solution of the compound (0.1 g, 0.2 mmol) dissolved in THF (5 ml) was added dropwise into the above suspension. The mixture was continuously stirred at room temperature for 72 h, filtered and concentrated in vacuum. The precipitate was washed with distilled water several times and then filtered and purified by column chromatography to obtain a red solid; yield, 50%.
1H NMR (400 MHz, Acetone): δ = 8.88 (d, J = 4.56 Hz, 4H, N-H), 8.18 (d, J = 9.2 Hz, 2H, Ar-H), 8.11 (d, J = 7.92 Hz, 2H, Ar-H), 8.02 (t, J = 4.68 Hz, 4H, N-H), 7.57 (t, J = 7.4 Hz, 2H, Ar-H), 7.50 (d, J = 8.36 Hz, 2H, Ar-H), 7.42 (d, J = 8.8 Hz, 4H, Ar-H), 7.35 (m, 4H, Ar-H), 7.14 (d, J = 8.76 Hz, 4H, Ar-H).
IR (thin film) νmax: 2969, 1744, 1596, 1489, 1408, 1263, 1220, 1061 and 749 cm−1.
13C NMR (CDCl3) δ = 163.27, 151.88, 150.83, 150.49, 148.20, 137.68, 136.85, 134.59, 134.18, 130.91, 129.28, 128.84, 128.17, 127.89, 127.53, 126.89, 124.11, 123.32, 121.76, 114.15, 77.33, 77.01, 76.69, 65.57, 30.58, 19.19, 13.73.
High resolution MS (M + H) calcd for C44H28N6O4: 705.2200, found: 705.2249.
Synthesis of H-bonded proton donors
(S)-4′-(Octan-2-yloxy)-[1,1′-biphenyl]-4-carbonitrite. Under the protection of nitrogen, we dissolved 4′-hydroxy-4-biphenylcarbonitrile (4.88 g, 25 mmol), (R)-(−)-2-octanol (3.25 g, 25 mmol) and triphenylphosphine (8.36 g, 27.5 mmol) in dry THF (50 ml), cooled to 0 °C using an ice-bath and slowly added DIAD (7.6 g, 0.0375 mmol) dissolved in THF, then stirred at room temperature for 12 hours. After removing bulk solvents, the orange oily mixture was purified by column chromatography to get a colorless oil; yield, 85%.
1H NMR (400 MHz, CDCl3): δ = 7.70-7.64(m, 4H, Ar-H), 7.55(d, J = 8.72 Hz, 2H, Ar-H), 7.01(d, J = 8.72 Hz, 2H, Ar-H), 4.45(m, 1H, -OCH), 1.63(m, 3H, -OCHCH3), 1.36–1.33 (m, 10H, CH2), 0.92(t, J = 6.56 Hz, 3H, CH3).
IR (thin film) νmax: 2929, 2855, 2225, 1602, 1520, 1492, 1246, 1178 and 821 cm−1.
Compound 3 was synthesized following similar procedures mentioned above; yield, 87%.
1H NMR (400 MHz, CDCl3): δ = 7.70–7.64(m, 4H, Ar-H), 7.54(d, J = 9.12 Hz, 2H, Ar-H), 7.01(d, J = 8.76 Hz, 2H, Ar-H), 4.45(m, 1H, -OCH), 1.62(m, 3H, -OCHCH3), 1.36–1.33(m, 10H, CH2), 0.92(t, J = 6.92 Hz, 3H, CH3).
IR (thin film) νmax: 2929, 2855, 2225, 1602, 1520, 1492, 1246, 1178 and 821 cm−1.
(S)-4′-(Octan-2-yloxy)-[1,1′-biphenyl]-4-carboxylic acid. Under the protection of nitrogen, (S)-4′-(octan-2-yloxy)-[1,1′-biphenyl]-4-carbonitrite (3.08 g, 10 mmol), 40 ml of water, 40 ml of ethanol and aqueous solution of KOH (0.1 g/10 ml) were introduced into the stirring apparatus. The mixture was heated under reflux and allowed to react at this temperature for about 10 hours. After being cooled to room temperature, the mixture was neutralized by addition of hydrochloric acid until pH = 7. The water was then distilled off in vacuum. The residue was taken up in ethanol and the insoluble salts were filtered off. The solvent of the filtrate was removed in vacuum to get a white powder, yield, 89%.
1H NMR (400 MHz, DMSO): δ = 12.92(s, 1H, Ar-COOH), 7.94(d, J = 8.32 Hz, 2H, Ar-H), 7.68(m, 4H, Ar-H), 7.02(m, 2H, Ar-H), 4.50(m, 1H, -OCH), 1.66(m, 3H, -OCHCH3), 1.40–1.25(m, 10H, CH2), 0.86(t, J = 6.48 Hz, 3H, CH3).
IR (thin film) νmax: 3201, 2928, 2857, 1603, 1524, 1246, 1192, 831 and 776 cm−1.
(R)-4′-(Octan-2-yloxy)-[1,1′-biphenyl]-4-carboxylic acid. (R)-OyBp was synthesized following similar procedures mentioned above; yield, 80%.
1H NMR (400 MHz, DMSO): δ = 12.94(s, 1H, Ar-COOH), 7.94(d, J = 8.28 Hz, 2H, Ar-H), 7.68(m, 4H, Ar-H), 7.01(m, 2H, Ar-H), 4.50(m, 1H, -OCH), 1.67(m, 3H, -OCHCH3), 1.42–1.25(m, 10H, CH2), 0.86(t, J = 6.44Hz, 3H, CH3).
IR (thin film) νmax: 3208, 2928, 2857, 1603, 1523, 1245, 1192, 831 and 776 cm−1.
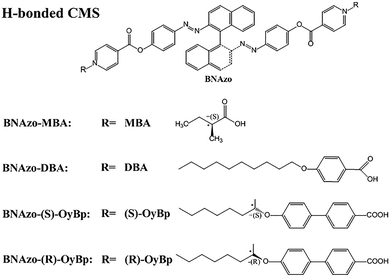
Preparation of H-bonded chiral molecular switches. H-bonded CMS were obtained by mixing the proton acceptor (BNAzo) with four proton donors (MBA, DBA, (S)-OyBp and (R)-OyBp) in a molar ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in THF respectively, followed by slow evaporation. Fig. 1 shows the chemical structures of four proton donors (MBA, DBA, (S)-OyBp and (R)-OyBp) and the corresponding H-bonded CMS (BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp). Then we doped these H-bonded CMS with the N-LC host (SLC1717) to get Ch-LCs with the light and temperature dual stimuli-responsive behavior, the Ch-LC molecular arrangements and the corresponding switching mechanisms are demonstrated in Fig. 2.
2 in THF respectively, followed by slow evaporation. Fig. 1 shows the chemical structures of four proton donors (MBA, DBA, (S)-OyBp and (R)-OyBp) and the corresponding H-bonded CMS (BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp). Then we doped these H-bonded CMS with the N-LC host (SLC1717) to get Ch-LCs with the light and temperature dual stimuli-responsive behavior, the Ch-LC molecular arrangements and the corresponding switching mechanisms are demonstrated in Fig. 2.
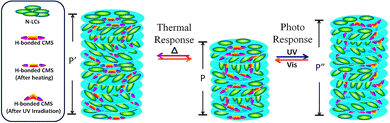 | ||
| Fig. 2 Schematic of the helical superstructure and the corresponding switching mechanism of H-bonded CMS in a N-LC host reversibly dynamically tuned by UV/vis light and heat. | ||
Measurements. The H-bonded CMS were characterized by FT-IR spectroscopy and variable-temperature FT-IR spectroscopy. The FT-IR spectra were recorded on a Nicolet 5700 spectrometer at frequencies ranging from 400 to 4000 cm−1. The samples were observed by POM (Leica DM2500P) with a hot stage calibrated with an accuracy of ±0.1 °C (Linkam, THBS-600). The reflection spectra were examined on an AvaSpec-2048 spectrophotometer in reflection mode. UV-vis and CD spectra of H-bonded CMS (in 1,4-dioxane) were recorded using a JASCO J810 spectrometer and a JASCO V550 spectrometer, respectively. UV irradiation was carried out using a mercury arc lamp (Powerarc UV 100) through a filter at 365 nm.
Results and discussion
In this study, the H-bonded CMS (BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp) were obtained by the self-assembly method. The effects of the configuration of chiral centers and introduction of proton donors on HTP of the H-bonded CMS were addressed in detail. Firstly, we used FT-IR spectrometry to distinguish the existence and intensity of H-bonding in this work. There are two strong H-bond self-assembly processes in our system, including the –OH⋯N between proton donors and acceptors and the –OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– between carboxylic acid dimers. Since the former being relatively stronger than the latter,19 given the appropriate proportion of donors and acceptors, the chiral pyridine derivative molecules are prone to combine with carboxylic acid molecules instead of the formation of carboxylic acid dimers. Fig. 3 shows the FT-IR spectra of the H-bonded CMS and their precursors. The FT-IR spectra confirm the presence of H-bonding obtained by the self-assembly process. As shown in Fig. 3, two peaks at around 2500 and 1900 cm−1 indicate the presence of hydrogen bonds, which almost coincides with the position of –OH stretching of H-bonds between the carboxylic acid and pyridyl groups. Fig. 3a shows that the intensity of peaks at 2720 and 2545 cm−1 decreased, which means the H-bonds between –OH⋯N have taken place for the bonds of MBA dimers. Similarly, such phenomenon can also be observed in BNAzo-DBA, BNAzo-(S)-OyBp, BNAzo-(R)-OyBp as shown in Fig. 3b–d indicating that four H-bonded CMS molecules with different proton donors were successfully assembled.
C– between carboxylic acid dimers. Since the former being relatively stronger than the latter,19 given the appropriate proportion of donors and acceptors, the chiral pyridine derivative molecules are prone to combine with carboxylic acid molecules instead of the formation of carboxylic acid dimers. Fig. 3 shows the FT-IR spectra of the H-bonded CMS and their precursors. The FT-IR spectra confirm the presence of H-bonding obtained by the self-assembly process. As shown in Fig. 3, two peaks at around 2500 and 1900 cm−1 indicate the presence of hydrogen bonds, which almost coincides with the position of –OH stretching of H-bonds between the carboxylic acid and pyridyl groups. Fig. 3a shows that the intensity of peaks at 2720 and 2545 cm−1 decreased, which means the H-bonds between –OH⋯N have taken place for the bonds of MBA dimers. Similarly, such phenomenon can also be observed in BNAzo-DBA, BNAzo-(S)-OyBp, BNAzo-(R)-OyBp as shown in Fig. 3b–d indicating that four H-bonded CMS molecules with different proton donors were successfully assembled.
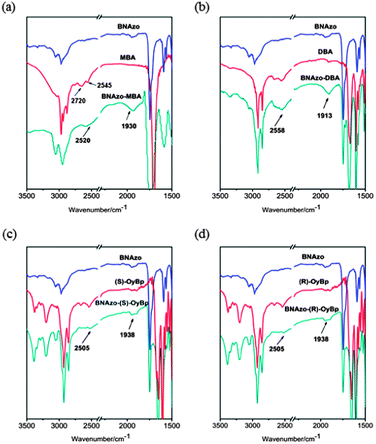 | ||
| Fig. 3 FT-IR spectra of the H-boned CMS and their precursors (a): BNAzo-MBA, BNAzo, MBA; (b): BNAzo-DBA, BNAzo, DBA; (c): BNAzo-(S)-OyBp, BNAzo, (S)-OyBp; and (d): BNAzo-(R)-OyBp, BNAzo, (R)-OyBp. | ||
In Fig. 4, the thermal stability of the H-bonded CMS was studied by the variable-temperature FT-IR spectroscopy. With the increase in temperature, the peaks indicating the H-bonds between the –OH and pyridyl groups (at around 2500 and 1900 cm−1) became weaker (as the inset shows), which means the elevation of temperature affected the interaction between proton donors and acceptors. We can also find that the four groups of H-bonded CMS (BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp) have good thermal stability across a temperature range of 30 °C to 110 °C, which sufficiently satisfies our experimental conditions.
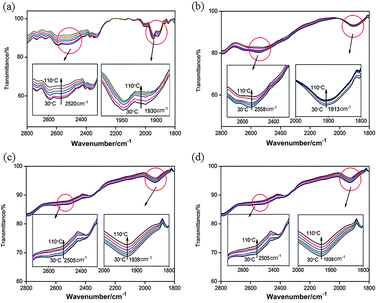 | ||
| Fig. 4 Variable-temperature FT-IR spectra of H-bonded CMS (a): BNAzo-MBA; (b): BNAzo-DBA, (c): BNAzo-(S)-OyBp, and (d): BNAzo-(R)-OyBp. | ||
In our study, the azobenzene moiety was introduced, which can be a photoresponsive part in H-bonded CMS. The effect of UV irradiation on the four H-bonded CMS (in 1,4-dioxane) was studied by circular dichroism (CD) spectroscopy and UV-vis spectroscopy. Fig. 5 and 6 show the change in UV and CD spectra of BNAzo-MBA (a), BNAzo-DBA (b), BNAzo-(S)-OyBp (c) and BNAzo-(R)-OyBp (d) under various UV irradiation conditions. Studies show that the trans form of azobenzene in BNAzo exhibited absorption maxima at 360 nm due to a π–π* transition and at 440 nm due to an n–π* transition.9f,20,21 It is found that after photoirradiation at 365 nm (2 mW cm−2), there is a decrease in the π–π* band and a relatively small amount of increase in the n–π* band, which indicates that trans–cis photoisomerization of BNAzo takes place on photoirradiation. Meanwhile, as shown in Fig. 6, CD spectra exhibit distinct bands for n–π* and π–π* transitions of the azochromophore. For example, in the initial state, BNAzo-MBA shows a negative band and a positive band for the π–π* transitions at 320 nm and 380 nm, respectively, followed by a relatively weak negative band at 450 nm, upon UV irradiation (365 nm, 2 mW cm−2), with the influence of photoisomerization of azobenzene moieties, we can find a gradual increase at 450 nm due to the formation of a cis isomer, and the pattern of the CD Cotton effect between 300 and 400 nm becomes weak due to the π–π* transitions, which corresponds to the phenomenon we observed in UV-vis spectra. Moreover, to prove that the photoisomerization of azobenzene has no impact on the thermoresponsive behavior in this work, we measured the change in absorption of four H-bonded CMS in 1,4-dioxane at various temperatures. Nearly no change is found in the UV-vis absorption spectra, which indicates that photoisomerization of the azobenzene structure does not take place during heat treatment process, therefore, the light and temperature switching processes of H-bonded CMS are two separative processes.
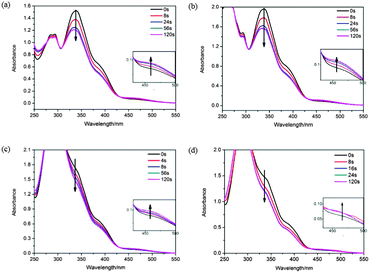 | ||
| Fig. 5 UV-vis absorption spectra of H-bonded CMS in 1,4-dioxane under UV irradiation (a): BNAzo-MBA; (b): BNAzo-DBA, (c): BNAzo-(S)-OyBp, (d): BNAzo-(R)-OyBp. | ||
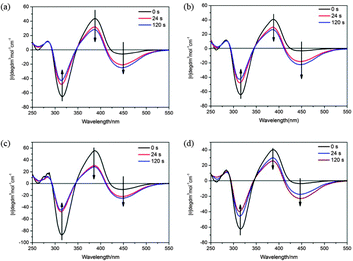 | ||
| Fig. 6 CD spectra of H-bonded CMS in 1,4-dioxane under UV irradiation (a): BNAzo-MBA; (b): BNAzo-DBA, (c): BNAzo-(S)-OyBp, (d): BNAzo-(R)-OyBp. | ||
Furthermore, we studied photo- and thermoresponsive behavior of H-bonded CMS in the commercially available N-LC host (SLC1717) by using the Grandjean-Cano method. The HTP value was used to characterize the ability of a chiral dopant for twisting the cholesteric mesophase and it is defined as the value of β and expressed by the following equation: β = 1/Pc, where P is the helical pitch and c is the concentration of the chiral dopant. Four LC samples were prepared by mixing the H-bonded CMS with SLC1717 in CH2Cl2, followed by the removal of solvent, the weight ratios of which correspond to the chiral dopant (BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SLC1717 host = 1
SLC1717 host = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
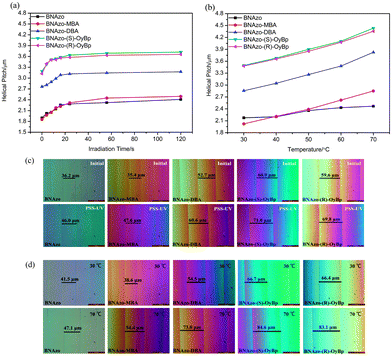
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 respectively. Moreover, to further discuss how the introduction of proton donors impacts the phototunability of the proton acceptor (BNAzo) and to make sure the H-bonds really contribute to the temperature dependence of the HTP value of Ch-LCs, we also prepared samples by mixing BNAzo (1 wt%) with SLC1717. Then we put the mixtures into wedges cell, which had been coated with polyimide and rubbed to align LC moieties. Owing to the twisting ability of H-bonded CMS, the N-LC host is induced to exhibit a helical structure so we can see Cano lines by using POM (Fig. 7c and d). The phototunable behavior of the LC host was observed by measuring the change in distance between Cano lines under UV irradiation. As Fig. 7c shows, the distance between Cano lines of BNAzo-(S)-OyBp is 60.9 μm in the initial state and it increases to 71 μm after irradiation with UV light, and the other four Ch-LC samples show the same trend during the photoisomerization process. Using the Cano wedge method, the pitch length can be determined according to the equation: p = 2Rtan
99 respectively. Moreover, to further discuss how the introduction of proton donors impacts the phototunability of the proton acceptor (BNAzo) and to make sure the H-bonds really contribute to the temperature dependence of the HTP value of Ch-LCs, we also prepared samples by mixing BNAzo (1 wt%) with SLC1717. Then we put the mixtures into wedges cell, which had been coated with polyimide and rubbed to align LC moieties. Owing to the twisting ability of H-bonded CMS, the N-LC host is induced to exhibit a helical structure so we can see Cano lines by using POM (Fig. 7c and d). The phototunable behavior of the LC host was observed by measuring the change in distance between Cano lines under UV irradiation. As Fig. 7c shows, the distance between Cano lines of BNAzo-(S)-OyBp is 60.9 μm in the initial state and it increases to 71 μm after irradiation with UV light, and the other four Ch-LC samples show the same trend during the photoisomerization process. Using the Cano wedge method, the pitch length can be determined according to the equation: p = 2Rtan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where R represents the distance between the Cano lines and θ is the wedge angle (θ = 1.5°, tan
θ, where R represents the distance between the Cano lines and θ is the wedge angle (θ = 1.5°, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.0262). The helical pitch length of these H-bonded CMS increase upon UV irradiation from the initial state to the photostationary state (PSS-UV), which means the HTP values of the H-bonded CMS decrease because of the trans to cis photoisomerization. For instance, before UV light irradiation at 365 nm, the pitch length of BNAzo-MBA is 1.85 μm while after UV irradiation for 120 s, the length of the helical pitch increases to 2.49 μm due to the photoisomerization process of azobenzene moieties, which correspond to the change in the distance between Cano lines as discussed above. As mentioned above, upon UV irradiation, photoisomerization of a rod shaped trans isomer to a bent cis isomer happens. In our study, it took less than two minutes for samples to reach the photostationary state under UV illumination. In Fig. 7a, we can find that among four H-bonded CMS, the initial helical pitch length of BNAzo-MBA is the lowest, which is similar to that of BNAzo and followed by BNAzo-DBA while those of BNAzo-(S)-OyBp and BNAzo-(R)-OyBp are the highest, suggesting that HTP of BNAzo-MBA is the largest and those of BNAzo-(S)-OyBp and BNAzo-(R)-OyBp are the smallest. According to the equation we mentioned above, the corresponding change in HTP values are summarized in Table 1, when phototunability of H-bonded CMS in the N-LC host was measured. As shown in Table 1, the HTP value of BNAzo-MBA is the highest while BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have the lowest HTP values. It is noteworthy that the change in the HTP value of BNAzo and BNAzo-MBA is also the biggest. In order to explain the effects of molecular structure on the HTP of H-bonded, the optimized structures predicted by Gaussian 03 calculations at the B3LYP/6-31G(d) level will be discussed. Fig. 8 shows the optimized structures of the chiral switches before and after UV irradiation. The molecular structures can be observed more visually by the optimized geometries. From an overall perspective, BNAzo-MBA is much smaller in molecular size than BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp and we can also find that BNAzo-MBA possesses a rod shape, while BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have a bent shape. Furthermore, we employ the molecular aspect ratio based on the molecular simulations to verify the HTP difference in H-bonded CMS. As demonstrated, the molecular aspect ratio, defined as the ratio of the molecular length (L) to the molecular diameter (D) is estimated from the model calculation and is one of the important parameters contributing to reveal the structures of the molecules.10d,e Investigation has been reported that a chiral dopant will have larger twisting power when it is similar in structure to the host LC molecules.10f The LC host we used here is SLC1717, a mixture of several rod-shaped compounds. Therefore, we can expect that a chiral dopant with a larger aspect ratio will exhibit a larger twisting power, because a larger L/D means the molecule is more rod-like. As listed in Fig. 8, the results reveal that the L/D of the H-bonded CMS molecules plays a relevant role in impacting their HTPs, which coincides with our previous study.18 For example, BNAzo-MBA exhibits the highest initial HTP and it has the largest molecular aspect ratio among these H-bonded CMS. BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have the lowest initial HTP values and their aspect ratio are also the lowest. Besides, the decrease of HTP of CMS after UV irradiation can also be explained by the optimized structures predicted by the B3LYP/6-31G(d) level and the molecular aspect ratio. Fig. 8 shows that the aspect ratio decreased after UV irradiation, which means the cis form has a greater bent degree than the trans form and the HTP value became smaller during the photoisomerization accordingly. However, it is worth mentioning that the change in HTP we obtained through the Cano-wedge method seems to be inconsistent with the aspect ratio based on the molecular simulation. We believe that it might be attributed to the difference between the molar mass of the four groups of CMS. For example, BNAzo-MBA has the lowest molar mass (908) while the molar mass of BNAzo-DBA is 1260. Since we used a constant weight fraction in our study, it means that the molar concentration is the highest for the mixture with the H-bonded CMS of the lowest molar mass. That might be the reason why the change in L/D of BNAzo-MBA is the lowest but its change in HTP is the highest. Moreover, although BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have the same molecular characteristics except for the opposite chirality, the HTP values measured did not differ significantly between them, which indicates that the tetrahedral chirality of two different donors is less efficient in comparison to much more efficient axial chirality of BNAzo.13
θ = 0.0262). The helical pitch length of these H-bonded CMS increase upon UV irradiation from the initial state to the photostationary state (PSS-UV), which means the HTP values of the H-bonded CMS decrease because of the trans to cis photoisomerization. For instance, before UV light irradiation at 365 nm, the pitch length of BNAzo-MBA is 1.85 μm while after UV irradiation for 120 s, the length of the helical pitch increases to 2.49 μm due to the photoisomerization process of azobenzene moieties, which correspond to the change in the distance between Cano lines as discussed above. As mentioned above, upon UV irradiation, photoisomerization of a rod shaped trans isomer to a bent cis isomer happens. In our study, it took less than two minutes for samples to reach the photostationary state under UV illumination. In Fig. 7a, we can find that among four H-bonded CMS, the initial helical pitch length of BNAzo-MBA is the lowest, which is similar to that of BNAzo and followed by BNAzo-DBA while those of BNAzo-(S)-OyBp and BNAzo-(R)-OyBp are the highest, suggesting that HTP of BNAzo-MBA is the largest and those of BNAzo-(S)-OyBp and BNAzo-(R)-OyBp are the smallest. According to the equation we mentioned above, the corresponding change in HTP values are summarized in Table 1, when phototunability of H-bonded CMS in the N-LC host was measured. As shown in Table 1, the HTP value of BNAzo-MBA is the highest while BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have the lowest HTP values. It is noteworthy that the change in the HTP value of BNAzo and BNAzo-MBA is also the biggest. In order to explain the effects of molecular structure on the HTP of H-bonded, the optimized structures predicted by Gaussian 03 calculations at the B3LYP/6-31G(d) level will be discussed. Fig. 8 shows the optimized structures of the chiral switches before and after UV irradiation. The molecular structures can be observed more visually by the optimized geometries. From an overall perspective, BNAzo-MBA is much smaller in molecular size than BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp and we can also find that BNAzo-MBA possesses a rod shape, while BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have a bent shape. Furthermore, we employ the molecular aspect ratio based on the molecular simulations to verify the HTP difference in H-bonded CMS. As demonstrated, the molecular aspect ratio, defined as the ratio of the molecular length (L) to the molecular diameter (D) is estimated from the model calculation and is one of the important parameters contributing to reveal the structures of the molecules.10d,e Investigation has been reported that a chiral dopant will have larger twisting power when it is similar in structure to the host LC molecules.10f The LC host we used here is SLC1717, a mixture of several rod-shaped compounds. Therefore, we can expect that a chiral dopant with a larger aspect ratio will exhibit a larger twisting power, because a larger L/D means the molecule is more rod-like. As listed in Fig. 8, the results reveal that the L/D of the H-bonded CMS molecules plays a relevant role in impacting their HTPs, which coincides with our previous study.18 For example, BNAzo-MBA exhibits the highest initial HTP and it has the largest molecular aspect ratio among these H-bonded CMS. BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have the lowest initial HTP values and their aspect ratio are also the lowest. Besides, the decrease of HTP of CMS after UV irradiation can also be explained by the optimized structures predicted by the B3LYP/6-31G(d) level and the molecular aspect ratio. Fig. 8 shows that the aspect ratio decreased after UV irradiation, which means the cis form has a greater bent degree than the trans form and the HTP value became smaller during the photoisomerization accordingly. However, it is worth mentioning that the change in HTP we obtained through the Cano-wedge method seems to be inconsistent with the aspect ratio based on the molecular simulation. We believe that it might be attributed to the difference between the molar mass of the four groups of CMS. For example, BNAzo-MBA has the lowest molar mass (908) while the molar mass of BNAzo-DBA is 1260. Since we used a constant weight fraction in our study, it means that the molar concentration is the highest for the mixture with the H-bonded CMS of the lowest molar mass. That might be the reason why the change in L/D of BNAzo-MBA is the lowest but its change in HTP is the highest. Moreover, although BNAzo-(S)-OyBp and BNAzo-(R)-OyBp have the same molecular characteristics except for the opposite chirality, the HTP values measured did not differ significantly between them, which indicates that the tetrahedral chirality of two different donors is less efficient in comparison to much more efficient axial chirality of BNAzo.13
| H-bonded chiral molecular switches | HTP (wt%)/μm−1 | Ä HTPa (μm−1) | * HTPb (%) | HTP (wt%)/μm−1 | Ä HTPc (μm−1) | * HTPb (%) | ||
|---|---|---|---|---|---|---|---|---|
| Initial | PSSUV | 30 °C | 70 °C | |||||
| a ΔHTP is calculated by subtracting the photostationary HTP value from the initial HTP value. b * HTP means the change in HTP is calculated by dividing ΔHTP by initial HTP. c ΔHTP is calculated by subtracting the 70 °C HTP value from the 30 °C HTP value. | ||||||||
| BNAzo | 52.7 | 41.5 | 11.2 | 21.3 | 46.0 | 40.5 | 5.5 | 11.9 |
| BNAzo-MBA | 53.9 | 40.1 | 13.8 | 25.6 | 49.4 | 35.1 | 14.3 | 34.7 |
| BNAzo-DBA | 36.2 | 31.5 | 4.7 | 13.0 | 35.0 | 26.1 | 8.9 | 25.3 |
| BNAzo-(S)-OyBp | 31.3 | 26.9 | 4.4 | 14.2 | 28.6 | 22.6 | 6.0 | 21.1 |
| BNAzo-(R)-OyBp | 32.0 | 27.3 | 4.7 | 14.6 | 28.7 | 23.0 | 5.7 | 20.0 |
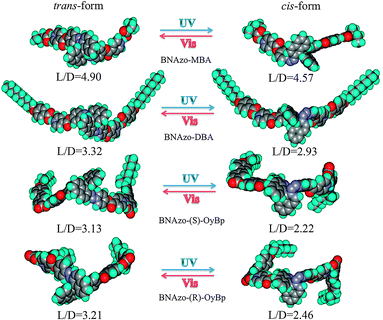 | ||
| Fig. 8 Optimized structures of trans and cis forms of BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp, and BNAzo-(R)-OyBp (space filling model) obtained by Gaussian 03 calculations at the B3LYP/6-31G(d) level. | ||
The thermoresponsive behavior of Ch-LCs based on the H-bonded CMS was studied in the same way. Fig. 7b shows that the helical pitch length of BNAzo, BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp increases as the temperature increases, in other words, HTP values of these H-bonded CMS decrease with the increase in temperature. This result may have the following explanations. First, this is mainly because of the change in the orientation parameter of the LC molecule induced by the weakened H-bonded interaction of H-bonded CMS.22 Secondly, other factors, such as the perturbation of the bulk LCs (SLC-1717) at high temperatures and the change in the binaphthyl twist angle induced by temperature (Table 1), may also contribute to the change in the HTP value. Meanwhile, we can also observe a similar change in the distance between Cano lines at various temperatures (Fig. 7d). The length of the helical pitch of BNAzo-(R)-OyBp was measured to be 3.48 μm at 30 °C and 4.35 μm at 70 °C, and for the BNAzo-(S)-OyBp, the pitch length increased from 3.49 μm (30 °C) to 4.43 μm (70 °C). According to the change in HTP values we summarized in Table 1, BNAzo-MBA has the most significant change in HTP value, it is possible that MBA has a more flexible ability to change the orientation of LC molecules in comparison to that of DBA, (R)-OyBp, and (S)-OyBp. Additionally, we could also find the change in HTP of BNAzo is 11.9%, which is smaller than changes in any other four Ch-LCs systems based on H-bonded CMS (34.7%, 25.3%, 21.1%, and 20.0%), so we can conclude that H-bonds between the carboxylic acid and pyridyl groups contribute to the temperature dependence of HTP in the H-bonded CMS.
As mentioned above, Ch-LCs can selectively reflect light, so we studied the temperature and irradiation time dependence of the selective reflection bands (SRB) of H-bonded CMS on the N-LC host. We prepared mixtures and the corresponding weight ratios are BNAzo-MBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SLC1717 = 5
SLC1717 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 and other three chiral dopants (BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp)
95 and other three chiral dopants (BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SLC1717 = 10
SLC1717 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
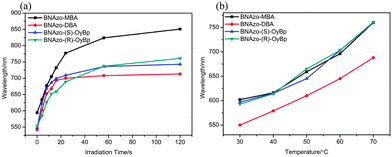
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90. Then we put the samples into glass cells. The glass cells were composed of two glass substrates, which were coated with a 3.0 wt% polyvinyl alcohol aqueous solution and subsequently rubbed with a textile cloth in one direction to generate a planar alignment. Fig. 9 shows the irradiation time and temperature dependence of the reflection bands of BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp respectively. As shown in Fig. 9a, the reflection bands of all the samples increased with the increase in irradiation time of UV light (365 nm, 6 mW cm−2) coinciding with the changes in HTP shown in Table 1. For example, the reflection wavelength of BNAzo-DBA experiences a red shift from 542 nm (0 s) to 713 nm (120 s) due to photoisomerization. Meanwhile, with the increase in temperature, the reflection wavelength of all the samples undergoes a red shift, which also corresponds to the results we discussed above. As shown in Fig. 9b, the reflection wavelength of BNAzo-(S)-OyBp increases from 597 nm (30 °C) to 759 nm (70 °C) attributed to the modulation of the intermolecular forces between the proton donors and acceptors as temperature increases.
90. Then we put the samples into glass cells. The glass cells were composed of two glass substrates, which were coated with a 3.0 wt% polyvinyl alcohol aqueous solution and subsequently rubbed with a textile cloth in one direction to generate a planar alignment. Fig. 9 shows the irradiation time and temperature dependence of the reflection bands of BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp respectively. As shown in Fig. 9a, the reflection bands of all the samples increased with the increase in irradiation time of UV light (365 nm, 6 mW cm−2) coinciding with the changes in HTP shown in Table 1. For example, the reflection wavelength of BNAzo-DBA experiences a red shift from 542 nm (0 s) to 713 nm (120 s) due to photoisomerization. Meanwhile, with the increase in temperature, the reflection wavelength of all the samples undergoes a red shift, which also corresponds to the results we discussed above. As shown in Fig. 9b, the reflection wavelength of BNAzo-(S)-OyBp increases from 597 nm (30 °C) to 759 nm (70 °C) attributed to the modulation of the intermolecular forces between the proton donors and acceptors as temperature increases.
Given the high HTPs as well as the remarkable variation in HTP values upon UV light irradiation and heat treatment, we filled a mixture of 8.1 wt% BNAzo-MBA in SLC1717 into a 10 μm thick planar aligned cell to observe the change in SRB of the Ch-LCs. The reflection central wavelength was at around 490 nm in the initial state. Upon UV irradiation, its reflection wavelength was tuned to 550 nm in only 30 s and reached a photostationary state in 60 s with a reflection central wavelength at around 700 nm (Fig. 10a). It is noteworthy that the process is reversible by visible light irradiation (450 nm, 15 mW cm−2) for 60 s or keeping it in the dark room at 50 °C for 2 h. Likewise, we can find that the reflection wavelength was tuned from 490 nm to 640 nm as the temperature increased from 25 °C to 65 °C and it is also thermally reversible. (Fig. 10b) Furthermore, four reflection colors (red, green, yellow and blue) can be observed in both glass cells filled with the same LC sample, suggesting that we have successfully fabricated LCs sensors that change the reflection color upon exposure to UV light as well as heat treatment by doping H-bonded CMS with an achiral N-LC host.
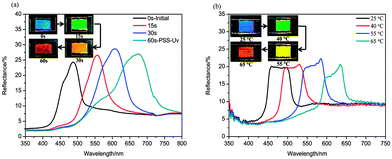 | ||
| Fig. 10 Reflection spectra of 8.1 wt% BNAzo-MBA in SLC1717 in 10 μm thick planar cells under UV irradiation (a) and at various temperatures (b). The insets show photographs of the cells. | ||
Conclusions
In summary, we fabricated four H-bonded chiral molecular switches (BNAzo-MBA, BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp), and photo/thermo-reversible tuning behavior of cholesteric liquid crystals based on the H-bonded chiral molecular switches was investigated. The reflection bands of all the cholesteric liquid crystal mixtures exhibited red shift under UV irradiation as well as with an increase of temperature. The isomerization of azobenzene moieties in H-bonded chiral molecular switches under UV/visible light is responsible for good phototunability. Meanwhile, the modulation of the interaction between H-bonded donors and acceptors with temperature plays one of the important roles for the temperature-sensitivity of the Ch-LCs. What is more, the optimized molecular structures obtained by Gaussian 03 calculations at the B3LYP/6-31G(d) level suggest that the introduction of rigid units such as the phenyl or biphenyl group in BNAzo-DBA, BNAzo-(S)-OyBp and BNAzo-(R)-OyBp can bend the molecular switches and lead to a difference in the HTP value of the four groups of the switches. Additionally, the HTP values of BNAzo-(S)-OyBp and BNAzo-(R)-OyBp are similar despite the opposite handedness of their proton donors, which is probably due to the less efficient tetrahedral chirality of two different donors in comparison to much more efficient axial chirality of BNAzo in our H-bonded self-assembled system.Acknowledgements
This research was supported by the National Natural Science foundation (Grant no. 51373013, 51173013 and 50903004) and Beijing Young Talents Plan (YETP0489). We also thank CHEMCLOUDCOMPUTING of Beijing University of Chemical Technology for the molecular simulation of this investigation.Notes and references
- (a) S. Pieraccini, S. Masiero, A. Ferrarini and S. G. Piero, Chem. Soc. Rev., 2011, 40, 258–271 RSC; (b) E. Sackmann, J. Am. Chem. Soc., 1971, 93, 7088–7090 CrossRef CAS; (c) A. Ryabchun, A. Bobrovsky, A. Sobolewska, V. Shibaev and J. Stumpe, J. Mater. Chem., 2012, 22, 6245–6250 RSC.
- (a) Q. Li, Y. Li, J. Ma, D.-K. Yang, T. J. White and T. J. Bunning, Adv. Mater., 2011, 23, 5069–5073 CrossRef CAS PubMed; (b) Y. Li, A. Urbas and Q. Li, J. Am. Chem. Soc., 2012, 134, 9573–9576 CrossRef CAS PubMed; (c) M. Mitov, Adv. Mater., 2012, 24, 6260–6276 CrossRef CAS PubMed.
- K. S. Burnham and G. B. Schuster, J. Am. Chem. Soc., 1998, 120, 12619–12625 CrossRef CAS.
- M. Mathews, R. S. Zola, D.-k. Yang and Q. Li, J. Mater. Chem., 2011, 21, 2098–2103 RSC.
- (a) C. Ruslim and K. Ichimura, J. Mater. Chem., 2002, 12, 3377–3379 RSC; (b) C. Xue, K. Gutierrez-Cuevas, M. Gao, A. Urbas and Q. Li, J. Phys. Chem. C, 2013, 117, 21603–21608 CrossRef CAS.
- S. A. Holmstrom, L. V. Natarajan, V. P. Tondiglia, R. L. Sutherland and T. J. Bunning, Appl. Phys. Lett., 2004, 85, 1949–1951 CrossRef CAS PubMed.
- (a) A. Bobrovsky, N. Boiko and V. Shibaev, Macromolecules, 2006, 39, 6367–6370 CrossRef CAS; (b) M. Teng, D. Wang, X. Jia, X. Fan, G. Kuang, X. Chen, D. Zou and Y. Wei, J. Phys. Chem. C, 2011, 115, 22540–22546 CrossRef CAS; (c) M. Mathews and N. Tamaoki, J. Am. Chem. Soc., 2008, 130, 11409–11416 CrossRef CAS PubMed; (d) H. Akiyama, A. Tanaka, H. Hiramatsu, J. i. Nagasawa and N. Tamaoki, J. Mater. Chem., 2009, 19, 5956 RSC.
- (a) Y. Wang, A. Urbas and Q. Li, J. Am. Chem. Soc., 2012, 134, 3342–3345 CrossRef CAS PubMed; (b) B. L. Feringa, J. Org. Chem., 2007, 72, 6635–6652 CrossRef CAS PubMed.
- (a) K. Rameshbabu, A. Urbas and Q. Li, J. Phys. Chem. B, 2011, 115, 3409–3415 CrossRef CAS PubMed; (b) T. van Leeuwen, T. C. Pijper, J. Areephong, B. L. Feringa, W. R. Browne and N. Katsonis, J. Mater. Chem., 2011, 21, 3142–3146 RSC; (c) H. Hayasaka, T. Miyashita, M. Nakayama, K. Kuwada and K. Akagi, J. Am. Chem. Soc., 2012, 134, 3758–3765 CrossRef CAS PubMed; (d) S. Pieraccini, G. Gottarelli, R. Labruto, S. Masiero, O. Pandoli and G. P. Spada, Chem. – Eur. J., 2004, 10, 5632–5639 CrossRef CAS PubMed; (e) Y. Li, M. Wang, T. J. White, T. J. Bunning and Q. Li, Angew. Chem., Int. Ed., 2013, 52, 8925–8929 CrossRef CAS PubMed; (f) L. Green, Y. Li, T. White, A. Urbas, T. Bunning and Q. Li, Org. Biomol. Chem., 2009, 7, 3930–3933 RSC; (g) B. S. Udayakumar and G. B. Schuster, J. Org. Chem., 1993, 58, 4165–4169 CrossRef CAS; (h) L.-M. Jin, Y. Li, J. Ma and Q. Li, Org. Lett., 2010, 12, 3552–3555 CrossRef CAS PubMed; (i) S. Pieraccini, S. Masiero, G. P. Spada and G. Gottarelli, Chem. Commun., 2003, 598–599 RSC.
- (a) Y. Yu, M. Nakano and T. Ikeda, Nature, 2003, 425, 145 CrossRef CAS PubMed; (b) K. Ichimura, S.-K. Oh and M. Nakagawa, Science, 2000, 288, 1624–1626 CrossRef CAS; (c) S. Kurihara, T. Yoshioka, T. Ogata, Z. A. Md and T. Nonaka, Liq. Cryst., 2003, 30, 1219–1223 CrossRef CAS; (d) T. Yoshioka and T. Ogata, Adv. Mater., 2005, 17, 1226–1229 CrossRef CAS; (e) M. Z. Alam, T. Yoshioka, T. Ogata, T. Nonaka and S. Kurihara, Liq. Cryst., 2007, 34, 1215–1219 CrossRef CAS; (f) C. Ruslim and K. Ichimura, Adv. Mater., 2001, 13, 37–40 CrossRef CAS.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed.
- Y. Li, A. Urbas and Q. Li, J. Org. Chem., 2011, 76, 7148–7156 CrossRef CAS PubMed.
- (a) M. Mathews, R. S. Zola, S. Hurley, D.-K. Yang, T. J. White, T. J. Bunning and Q. Li, J. Am. Chem. Soc., 2010, 132, 18361–18366 CrossRef CAS PubMed; (b) T. J. White, R. L. Bricker, L. V. Natarajan, N. V. Tabiryan, L. Green, Q. Li and T. J. Bunning, Adv. Funct. Mater., 2009, 19, 3484–3488 CrossRef CAS; (c) J. Ma, Y. Li, T. White, A. Urbas and Q. Li, Chem. Commun., 2010, 46, 3463–3465 RSC; (d) Y. Li and Q. Li, Org. Lett., 2012, 14, 4362–4365 CrossRef CAS PubMed; (e) Y. Wang and Q. Li, Adv. Mater., 2012, 24, 1926–1945 CrossRef CAS PubMed.
- (a) A. Demenev, S. H. Eichhorn, T. Taerum, D. F. Perepichka, S. Patwardhan, F. C. Grozema, L. D. A. Siebbeles and R. Klenkler, Chem. Mater., 2010, 22, 1420–1428 CrossRef CAS; (b) P. V. Shibaev, J. Madsen and A. Z. Genack, Chem. Mater., 2004, 16, 1397–1399 CrossRef CAS; (c) G. A. Shandryuk, S. A. Kuptsov, A. M. Shatalova, N. A. Plate and R. V. Talroze, Macromolecules, 2003, 36, 3417–3423 CrossRef CAS; (d) H. Kihara, T. Kato, T. Uryu and J. M. J. Fréchet, Chem. Mater., 1996, 8, 961–968 CrossRef CAS; (e) T. J. White, M. E. McConney and T. J. Bunning, J. Mater. Chem., 2010, 20, 9832–9847 RSC; (f) C. Ohm, M. Brehmer and R. Zentel, Adv. Mater., 2010, 22, 3366–3387 CrossRef CAS PubMed; (g) H. Yu and T. Ikeda, Adv. Mater., 2011, 23, 2149–2180 CrossRef CAS PubMed; (h) D. J. Broer, C. M. W. Bastiaansen, M. G. Debije and A. P. H. J. Schenning, Angew. Chem., Int. Ed., 2012, 51, 7102–7109 CrossRef CAS PubMed; (i) D. J. Mulder, A. P. H. J. Schenning and C. W. M. Bastiaansen, J. Mater. Chem. C, 2014, 2, 6695–6705 RSC.
- (a) F. J. Chen, J. B. Guo, Z. J. Qu and J. Wei, J. Mater. Chem., 2011, 21, 8574–8582 RSC; (b) F. J. Chen, J. B. Guo, O. Y. Jin and J. Wei, Chin. J. Polym. Sci., 2013, 31, 630–640 CrossRef CAS PubMed.
- (a) T. Kato and J. M. J. Frechet, J. Am. Chem. Soc., 1989, 111, 8533–8534 CrossRef CAS; (b) T. Kato, M. Fukumasa and J. M. J. Frechet, Chem. Mater., 1995, 7, 368–372 CrossRef CAS.
- N. Herzer, H. Guneysu, D. J. D. Davies, D. Yildirim, A. R. Vaccaro, D. J. Broer, C. W. M. Bastiaansen and A. P. H. J. Schenning, J. Am. Chem. Soc., 2012, 134, 7608–7611 CrossRef CAS PubMed.
- Y. Xie, D. Fu, O. Jin, H. Zhang, J. Wei and J. Guo, J. Mater. Chem. C, 2013, 1, 7346–7356 RSC.
- T. Kato, J. M. Frechet, P. G. Wilson, T. Saito, T. Uryu, A. Fujishima, C. Jin and F. Kaneuchi, Chem. Mater., 1993, 5, 1094–1100 CrossRef CAS.
- H. Goto and K. Kawabata, Polym. Chem., 2011, 2, 1098–1106 RSC.
- T. Ikeda, J. Mater. Chem., 2003, 13, 2037–2057 RSC.
- A. Takahashi, V. A. Mallia and N. Tamaoki, J. Mater. Chem., 2003, 13, 1582–1587 RSC.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |