A simple carbazole based sensitizer attached to a Nafion-coated-TiO2 photocatalyst: the impact of controlling parameters towards visible light driven H2 production†
Amritanjali
Kumari
a,
Indranil
Mondal
a and
Ujjwal
Pal
*ab
aDepartment of Chemistry and Biomimetics, CSIR-Central Mechanical Engineering Research Institute, M.G Avenue, Durgapur-713209, West Bengal, India. E-mail: upal03@gmail.com; amritanjali4@gmail.com; indra07.mondal@gmail.com; Fax: +91 343-2546745; Tel: +91 343-6452136
bIndia Network Institute of Solar Energy, (CSIR-NISE) and Academy of Scientific and Innovative Research (AcSIR), New Delhi, India
First published on 10th November 2014
Abstract
Here we report a new composite material consisting of a simple and cost effective carbazole based photosensitizer attached onto a Nafion/Pt/TiO2 (NPT) hybrid as a high-performance photocatalyst for H2 evolution under visible light. The engineered composite of dye with NPT is shown to render improvement in its photocatalytic activity. We have spectroscopically observed that the spacer free simple carbazole based dye was perfectly attached onto TiO2 surfaces, which also remained unaffected in alkaline medium. The development of the photocatalyst was confirmed by different morphological characterization procedures. Under optimum conditions (pH 10, 1.0 × 10−4 mol/10 mg AM dye, 0.5 wt% Pt), the maximal apparent quantum yield (AQY%) of D1@PT and D1@NPT for hydrogen evolution increases up to 16.5% and 19.16% under ∼2-sun-intensity of visible light irradiation with triethanolamine (TEOA) as a sacrificial agent. The combined effects of several factors may contribute to the remarkably enhanced photocatalytic activity for the D1@NPT sample including dye concentration, pH and catalyst amount variation, and the spacer effect of the sensitizers (with and without oligothiophene moieties).
1. Introduction
Photo-assisted water splitting is a promising method for H2 production which is one of the clean renewable energy sources. Since the publication of a report on photo induced water splitting on a TiO2 electrode under UV irradiation by Fujishima and Honda,1 enormous attention has been focused on photocatalytic H2 evolution over semiconductors.2 Owing to their many desirable properties,3 a large number of TiO2 based photocatalysts have been developed to date for effective H2 production via water splitting.4 However, the only drawback of the TiO2 semiconductor is that it absorbs a small portion of the solar spectrum in the UV region because of its wide band gap of 3.2 eV. Therefore, extensive efforts have been made to develop dye sensitized photocatalysts, capable of utilizing the less energetic but more abundant visible light.5 Dye modification has been used to make TiO2 active to visible-light excitation, and such modifications are usually performed by chemical bonding of a functional group (carboxylate or phosphonate) or through hydrogen bonding. In this particular process, electron injection occurs by excitation of the dye and consequent water reduction occurs at the co-catalyst site of the semiconductor which is usually Pt.6Metal free dyes have profoundly higher extent of light-harvesting capability than metal complexes because of their (i) high molar extinction coefficients, (ii) donor–pi–bridge–acceptor (D–π–A) and (iii) availability of versatile functional molecules associated with fine tuning of the electronic and chemical properties.7 Some organic dye sensitized TiO2 photocatalysts such as phenothiazine,5b xanthene,8 merocyanine9 and coumarin10 are also efficient for water splitting. However, their apparent quantum yields are not high enough in the visible region.
Recently, many researchers have been paying a good deal of attention to the carbazole based dyes for use in DSSCs due to their facile molecular tuning and high molar extinction coefficients.11 To the best of our knowledge, there is a single report, where Abe et al. have well established the two step photoexcitation in water splitting over a niobate semiconductor using a commercial coumarin and carbazole based sensitizer.2a However, an inexpensive method of photocatalytic H2 generation using organic dyes is still under sporadic investigation.
Due to the unique chemical structure and electronic properties, Nafion is widely used as a proton exchange membrane in the application of a fuel cell.12 Furthermore H. Park et al. have properly explained the immobilization of a component through Nafion in homogeneous photo-assisted H2 generation without using any semiconductor.13 Nafion was also used as the binding site for ruthenium based cationic dyes in sensitized H2 generation.14 However, as far as we are aware, the application of Nafion in stabilization of a sensitizer for enhanced photocatalytic H2 evolution is not yet reported.
Here, we report for the first time a simple carbazole based organic dye (Fig. 1) sensitized Nafion coated Pt/TiO2 system (D1@NPT) for photocatalytic H2 production under visible light irradiation. The as-prepared sensitizer molecule was developed using inexpensive synthesis methodology. This study also explores the significance of Nafion incorporation towards improved photocatalytic efficiency as D1@NPT shows a remarkably high TON of 7843. Moreover important operational parameters like dye concentration, photocatalyst dosage, effect of the activation temperature of the TiO2 and pH of the medium etc. were screened and H2 generation was optimized. Furthermore, the possible photocatalytic H2 production mechanism and the role of Nafion are also discussed.
 | ||
| Fig. 1 Molecular structure of (a) AM, (b) MK2, (c) eosin Y, (d) N719 dyes and (e) the Nafion polymer. | ||
2. Experimental section
9-Ethyl-9H-carbazol-3-carbaldehyde, anatase TiO2, chloroauric acid, chloroplatinic acid and MK2 dye were purchased from Sigma Aldrich. Nafion solution (D1020) was purchased from DuPont. Piperidine was purchased from Spectrochem. All the chemicals were of AR grade and used without further purification unless otherwise stated. Organic solvents were purified and dried before synthesis of the sensitizer.2.1. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA, 95
EA, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) on silica gel to obtain a pure compound as a yellowish green solid with 90% yield. 1H NMR (300 MHz, methanol-d4) δ 8.39 (1H, s), (1H, d, J = 7.7 Hz), 7.56–7.39 (4H, m), 7.17 (1H, d, J = 7.7 Hz), 7.34 (1H, t), 8.2 (1H, t), 4.42 (2H, q, J = 6.9 Hz), 1.40 (3H, t, J = 6.9 Hz), ESI-MS: MS (ESI†), m/z [M˙+ Na]: calcd: 313.10; found: 313.00 and FTIR (details in ESI†).
5) on silica gel to obtain a pure compound as a yellowish green solid with 90% yield. 1H NMR (300 MHz, methanol-d4) δ 8.39 (1H, s), (1H, d, J = 7.7 Hz), 7.56–7.39 (4H, m), 7.17 (1H, d, J = 7.7 Hz), 7.34 (1H, t), 8.2 (1H, t), 4.42 (2H, q, J = 6.9 Hz), 1.40 (3H, t, J = 6.9 Hz), ESI-MS: MS (ESI†), m/z [M˙+ Na]: calcd: 313.10; found: 313.00 and FTIR (details in ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mL).8 Then commercial TiO2 (cTiO2) was dispersed into the resulting solution and the mixture was kept under stirring conditions at room temperature in the dark for 24 hours. After completion of the reaction, it was filtered, washed with ethanol and then dried in the air. These final composites were labelled as AM@TiO2 (D1@T) and MK2@TiO2 (D2@T). The same method has been applied for preparation of dye anchored Au/TiO2, Pt/TiO2 and Nafion coated Pt/TiO2 composites and are labelled as D1@AT, D2@AT, D1@PT, D2@PT, D1@NPT and D2@NPT.
1 v/v, 10 mL).8 Then commercial TiO2 (cTiO2) was dispersed into the resulting solution and the mixture was kept under stirring conditions at room temperature in the dark for 24 hours. After completion of the reaction, it was filtered, washed with ethanol and then dried in the air. These final composites were labelled as AM@TiO2 (D1@T) and MK2@TiO2 (D2@T). The same method has been applied for preparation of dye anchored Au/TiO2, Pt/TiO2 and Nafion coated Pt/TiO2 composites and are labelled as D1@AT, D2@AT, D1@PT, D2@PT, D1@NPT and D2@NPT.
2.2. Photocatalytic experiment
The photocatalytic H2 generation experiments were carried out in a doubly jacketed Pyrex glass reactor with a flat optical window and an external cooling jacket. All experiments were carried out in 20 mL aqueous suspension containing 10 mg of the photocatalyst and 10 vol% of TEOA as SED. It was then air sealed with a rubber septum. Before light irradiation, the dissolved air was removed by keeping it for 20 min under high vacuum followed by purging of Ar gas. The Xenon arc lamp (400 W) was used as a light source. The reaction mixture was kept under constant stirring conditions during the course of irradiation and the resulting evolved gases were analyzed by gas chromatography using a Perkin Elmer Clarus 580 GC equipped with a molecular sieve 5 Å column, a thermal conductivity detector (TCD) and argon as carrier gas.3. Results and discussion
3.1. Characterization
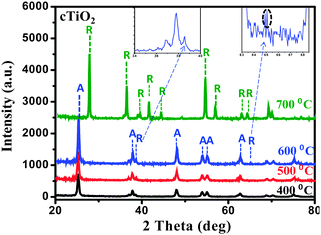
Optical properties of the dye and dye@TiO2 composites have been clarified on the basis of the UV-vis diffuse reflectance spectra (DRS). The electrochemical properties of the dyes have been studied by cyclic voltammetry (CV). To study the phase transformation, powder X-ray diffraction (XRD) of cTiO2 was carried out at different temperatures. FTIR spectra were recorded for dye and dye@TiO2 composites. The morphology of the sample was investigated by field emission scanning electron microscopy (FESEM); Nafion and gold deposition was confirmed by a high resolution transmission electron microscopy (HRTEM) study. Moreover, the as-synthesized dye (AM) was fully characterized by NMR, ESI-MS, FTIR and results have been incorporated in the ESI.† | ||
| Fig. 2 (a) UV-Vis spectra of AM and MK2 dye in chloroform (5 × 10−5 M), (b) cyclic voltammogram of AM dye traces at a scan rate of 50 mV s−1 measured in a 0.1 M [TBA][BF5] CH3CN solution. | ||
| Dyes | λ max (nm)/εmaxb (M−1 cm−1) | E 0–0 (eV) | E ox (V) | HOMOe (eV) | LUMOf (eV) |
|---|---|---|---|---|---|
| a Absorption. b Emission spectra of dyes. c E 0–0 was estimated from the transition energy measured at the onset of absorption spectra. d Oxidation potential of the dyes in CH3CN with 0.1 M tetrabutylammonium pentafluoroborate (TBABF5) as electrolyte. e The highest occupied molecular orbital (HOMO) value calculated using the potential value of oxidative waves. f The lowest unoccupied molecular orbital (LUMO) value was calculated by HOMO + E0–0. | |||||
| AM | 409/42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
3.03 | 1.95 | −6.45 | −3.42 |
| MK2 | 505/40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 203 203 |
2.07 | 0.96 | −5.05 | −2.98 |
The DRS of bare TiO2, Au/TiO2 and D2@AT were taken to study the changes in optical properties which have been incorporated in Fig. 3b. In Au/TiO2, a broad absorption band at around 575 nm was observed in the visible region, which was due to surface plasmon resonance (SPR) effects of the Au nanoparticle on the TiO2 surface.17 The plasmonic band came around 575 nm, attributed to the size of spherical Au nanoparticles of ∼15 nm, which creates an induced electric field onto the TiO2 surface.18 It is noteworthy that this dye sensitized system also takes advantage of the SPR effect towards hydrogen production which cannot be found in the other reported photocatalytic systems. After being sensitized with the dye, the whole composite shows a broad absorption over a wide spectral range from 400–700 nm which is attributed to the improved light harvesting nature of the photocatalyst composites.
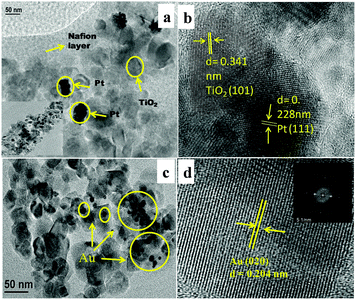 | ||
| Fig. 5 (a) TEM images of Nf/Pt/TiO2, (b) HRTEM of Nf/Pt/TiO2, (c) TEM images of AuTiO2 and (d) HRTEM of Au deposited on TiO2. | ||
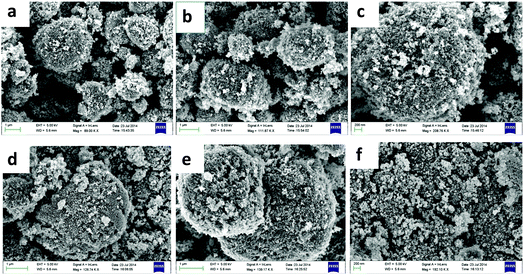
Fig. 6 shows the FESEM images of Pt/TiO2 and Nf/Pt/TiO2 composites. Pt/TiO2 (Fig. 6a–c) exhibited mesoporous microspheres morphology with unsymmetrical interior. Similar morphology was found in the Nf/Pt/TiO2 composite (Fig. 6d–f) though a Nafion layer was not visible in the image.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is present in both AM and MK2 dye and disappeared after being sensitized on the TiO2 surface. It is interesting to note that peaks at 1380 and 1381 cm−1 for D1@T and D2@T composites respectively appeared due to the –COO− group, which corroborates the formation of ester-like linkage of the carboxyl group between dye and TiO2.24
O is present in both AM and MK2 dye and disappeared after being sensitized on the TiO2 surface. It is interesting to note that peaks at 1380 and 1381 cm−1 for D1@T and D2@T composites respectively appeared due to the –COO− group, which corroborates the formation of ester-like linkage of the carboxyl group between dye and TiO2.24
3.2. Photocatalytic H2 evolution
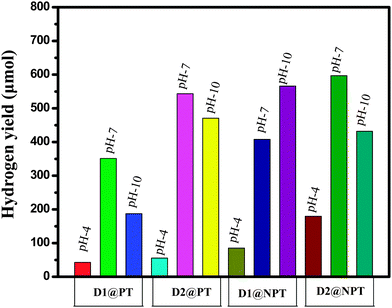
The photocatalytic activity of the catalyst was analyzed by the H2 evolution over the prepared spacer free and oligothiophene spacer dye sensitized under visible light irradiation. The experiments were carried out over 20 mL of aqueous suspension containing 10 mg of the photocatalyst and 10 vol% of TEOA as SED. In the whole experiment, important operational parameters like dye concentration, the catalyst amount, the pH effect, loading of different co-catalysts, activation temperature of TiO2 were thoroughly screened. It is important to note that all the experiments were carried out at room temperature (rt) and pH was adjusted by addition of 1 (M) HCl. During irradiation, the colour of aqueous suspension changed from red to yellow. For comparison, sensitizing effects of two commercial dyes EY and N719 have also been experimented under the specified conditions. These are presented in Fig. 7 and results have been shown in Table 2.| Catalyst | H2 yield (μmol) | Rate (μmol h−1) | TON | AQY (%) |
|---|---|---|---|---|
| a Dye sensitized TiO2 composites after 6 h of irradiation under visible light (λ ≥ 400 nm), conditions: 10 mg of the photocatalyst (0.1 μmol dye) in 20 mL of 10 vol% neutral aqueous TEOA (2 mL) solution. | ||||
| D1@T | 39.6 | 6.6 | 761 | 1.86 |
| D2@T | 42.4 | 7.0 | 815 | 1.99 |
| D1@AT | 127.3 | 21.2 | 2448 | 5.98 |
| D2@AT | 176.1 | 29.3 | 3386 | 8.27 |
| D1@PT | 351.1 | 58.5 | 6751 | 16.5 |
| D2@PT | 470.7 | 78.4 | 9051 | 22.12 |
| D1@NPT | 407.87 | 67.9 | 7843 | 19.16 |
| D2@NPT | 566.9 | 94.4 | 10901 | 26.64 |
| EY@PT | 20.49 | 3.41 | 419 | 0.96 |
| N719@PT | 8.15 | 1.35 | 163 | 0.38 |
The apparent quantum yield was estimated by the following equation:
 | (i) |
3.3. Mechanistic pathway
We have proposed a mechanism based on the above results of photocatalytic H2 production in D1@PT and D1@NPT systems in Scheme 2. The excitation of sensitizer was initiated upon visible light irradiation, where the photo-excited sensitizer transfers an electron from the HOMO of the dye to its LUMO which leads to the excitation of the dye molecule as D* (process 1). The oxidized dye species forms after electron injection into the TiO2 CB and is subsequently stabilized by the Nafion layer (processes 2 and 3). Processes 4 and 5 describe the trapping of electrons by Pt. Thereafter the injected electrons reduce the water molecule at the Pt site (process 6). The dye regenerates via taking up of electrons from SED and drives the photocatalytic process in a repeated manner (process 7).4. Conclusions
In conclusion, a new highly efficient photocatalyst containing a carbazole based photosensitizer and Nafion coated Pt/TiO2 is synthesized and its efficacy for visible-light photocatalytic water splitting is reported. Following a systematic investigation, the hybrid photocatalyst D1@NPT showed a highest turnover number of 7843 and the apparent quantum yield reached 19.16%. The sensitizer used in the hybrid composite is simple and also cost effective compared to many reported dyes (MK2, N719 etc.) for hydrogen production from water. Further, the results of the present study showed that the spacer group with an extended π-spacer linker to the carbazole sensitizer increases the light-harvesting ability and photocatalytic activity of the Nf/Pt/TiO2 composite at neutral pH. According to comprehensive analysis, it can be concluded that the improved photocatalytic activity is due to the combined effects of several factors, including the synergistic effect of Nafion-TiO2, strong visible light absorption of hybrid systems D1@NPT and D2@NPT and an anticipated low recombination rate. This work provides a favourable approach with a desired synergy to efficiently harness solar energy for renewable hydrogen production.Acknowledgements
The authors acknowledge the solar energy network project TAPSUN, NWP-56 for the financial support and Prof. Dr P. Pal Roy Director of CSIR-CMERI, for his endless encouragement in research. Amritanjali Tiwari and Indranil Mondal acknowledge AcSIR for PhD enrollment.References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS
.
-
(a) R. Abe, K. Shinmei, N. Koumura, K. Hara and B. Ohtani, J. Am. Chem. Soc., 2013, 135, 16872 CrossRef CAS PubMed
; (b) L. Qi, J. Yu and M. Jaroniec, Phys. Chem. Chem. Phys., 2011, 13, 8915 RSC
; (c) Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575 CrossRef CAS PubMed
.
-
(a) A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS
; (b) O. Carp, C. L. Huisman and A. Reller, Prog. Solid State Chem., 2004, 32, 33 CrossRef CAS PubMed
; (c) M. Pera-Titus, V. Garcia-Molina, M. A. Banos, J. Gimenez and S. Esplugas, Appl. Catal., B, 2004, 47, 219 CrossRef CAS PubMed
; (d) D. Chaterjee and S. Dasgupta, J. Photochem. Photobiol., C, 2005, 6, 186 CrossRef PubMed
; (e) X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed
; (f) U. I. Gaya and A. H. Abdullah, J. Photochem. Photobiol., C, 2008, 9, 1 CrossRef CAS PubMed
.
-
(a) W. D. K. Clark and N. Sutin, J. Am. Chem. Soc., 1977, 99, 4676 CrossRef CAS
; (b) M. T. Spitler and M. J. Calvin, J. Chem. Phys., 1977, 66, 4294 CrossRef CAS PubMed
; (c) A. Hamnett, M. P. Dare-Edwards, R. D. Wright, K. R. Seddon and J. B. Goodenough, J. Phys. Chem., 1979, 83, 3280 CrossRef CAS
; (d) M. P. Dare-Edwards, J. B. Goodenough, A. Hamnett, K. R. Seddon and R. D. Wright, Faraday Discuss. Chem. Soc., 1980, 70, 285 RSC
; (e) K. Zhang, Q. Liu, H. Wang, R. Zhang, C. Wu and J. R. Gong, Small, 2013, 9, 2452 CrossRef CAS PubMed
; (f) C. J. Li, G. R. Xua, B. Zhanga and J. R. Gong, Appl. Catal., B, 2012, 115–116, 201 CrossRef CAS PubMed
.
-
(a) M. Watanabe, H. Hagiwara, A. Iribe, Y. Ogata, K. Shiomi, A. Staykov, S. Ida, K. Tanaka and T. Ishihara, J. Mater. Chem., 2014, 2, 12952 RSC
; (b) J. Lee, J. Kwak, K. C. Ko, J. H. Park, J. H. Ko, N. Park, E. Kim, D. H. Ryu, T. K. Ahn, J. Y. Lee and S. U. Son, Chem. Commun., 2012, 48, 11431 RSC
; (c) W. J. Youngblood, S. H. A. Lee, K. Maeda and T. E. Mallouk, Acc. Chem. Res., 2009, 42, 1966 CrossRef CAS PubMed
.
-
(a) S. H. Lee, Y. Park, K. R. Wee, H. J. Son, D. W. Cho, C. Pac, W. Choi and S. O. Kang, Org. Lett., 2010, 12, 460 CrossRef CAS PubMed
; (b) E. A. Malinka, G. L. Kamalov, S. V. Vodzinskii, V. I. Melnik and Z. I. Zhilina, J. Photochem. Photobiol., A, 1995, 90, 153 CrossRef CAS
; (c) E. Bae, W. Choi, J. Park, H. S. Shin, S. B. Kim and J. S. Lee, J. Phys. Chem. B, 2004, 108, 14093 CrossRef CAS
.
- M. Marszalek, S. Nagane, A. Ichake, R. H. Baker, V. Paul, S. M. Zakeeruddin and M. Graetzel, J. Mater. Chem., 2012, 22, 889 RSC
.
- R. Abe, K. Shinmei, K. Hara and B. Ohtani, Chem. Commun., 2009, 3577 RSC
.
- X. Zhang, Z. Jin, Y. Li, S. Li and G. Lu, J. Phys. Chem. C, 2009, 113, 2630 CAS
.
- R. Abe, K. Sayama and H. Arakawa, Chem. Phys. Lett., 2002, 362, 441 CrossRef CAS
.
- Z. S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993 CrossRef CAS
.
- W. Kim, T. Seok and W. Choi, Energy Environ. Sci., 2012, 5, 6066 CAS
.
- H. Park, Y. Park, E. Bae and W. Choi, J. Photochem. Photobiol., A, 2009, 203, 112 CrossRef CAS PubMed
.
- H. Park and W. Choi, J. Phys. Chem. B, 2005, 109, 11667 CrossRef CAS PubMed
.
- C. G. Silva, R. J. Rez, T. Marino, R. Molinari and H. García, J. Am. Chem. Soc., 2011, 133, 595 CrossRef PubMed
.
- J. Park, J. Yi, T. Tachikawa, T. Majima and W. Choi, J. Phys. Chem. Lett., 2010, 1, 1351 CrossRef CAS
.
- D. Jose, C. M. Sorensen, S. S. Rayalu, K. M. Shrestha and K. J. Klabunde, Int. J. Photoenergy, 2013, 2013, 1 CrossRef PubMed
.
- J. C. S. Wu, J. J. Chen, P. C. Wu and D. P. Tsai, SPIE Newsroom, 2012 DOI:10.1117/2.1201201.004079
.
- N. Robertson and H. J. Snaith, Adv. Energy Mater., 2014, 1400166 Search PubMed
.
- N. Rungjaroentawon, S. Onsuratoom and S. Chavadej, Int. J. Hydrogen Energy, 2012, 37, 11061 CrossRef CAS PubMed
.
-
J. V. Smith, Am. Soc. Test. Mater., 1960 Search PubMed
.
- Z. Z. Jiang, Z. B. Wang, Y. Y. Chu, D. M. Gu and G. P. Yin, Energy Environ. Sci., 2011, 4, 728 CAS
.
- M. Zhu, B. Lei, F. Ren, P. Chen, Y. Shen, B. Guan, Y. Du, T. Li and M. Liu, Sci. Rep., 2014, 4, 5259, DOI:10.1038/srep05259
.
- K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2003, 107, 597 CrossRef CAS
.
- K. B. Dhanalakshmi, S. Latha, S. Anandan and P. Maruthamuthu, Int. J. Hydrogen Energy, 2001, 26, 669 CrossRef CAS
.
- J. Yu, H. Yu, B. Chenga and C. Trapalis, J. Mol. Catal. A: Chem., 2006, 249, 135 CrossRef CAS PubMed
.
-
P. Chowdhury, PhD thesis, School of Graduate and Postdoctoral Studies, The University of Western Ontario London, Onterio, Canada, 2012
.
- X. Liu, Y. Li, S. Peng, G. Lub and S. Li, Photochem. Photobiol. Sci., 2013, 12, 1903 CAS
.
- E. Reisner, J. C. Fontecilla-Camps and F. A. Armstrong, Chem. Commun., 2009, 550 RSC
.
- T. Sreethawong, C. Junbua and S. Chavadej, J. Power Sources, 2009, 190, 513 CrossRef CAS PubMed
.
- E. A. Malinka and G. L. Kamalov, J. Photochem. Photobiol., A, 1994, 81, 193 CrossRef CAS
.
- H. Dürr, S. Boßmann and A. Beuerlein, J. Photochem. Photobiol., A, 1993, 73, 233 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nj01436g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |