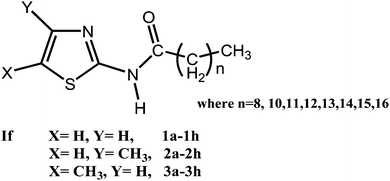
Odd–even effect in a thiazole based organogelator: understanding the interplay of non-covalent interactions on property and applications†
Priyanka
Yadav
and
Amar
Ballabh
 *
*
Department of Chemistry, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara 390002, Gujarat, India. E-mail: bamar.chem@gmail.com; amar.ballabh-chem@msubaroda.ac.in; Fax: +91-265-2795569; Tel: +91-265-2795552
First published on 4th November 2014
Abstract
New series of thiazole based amides, namely, 1e [N-(thiazol-2-yl)pentadecamide] to 1h [N-(thiazol-2-yl)stearamide], 2e [N-(4-methylthiazol-yl)pentadecamide] to 2h [N-(4-methylthiazol-yl)stearamide], 3e [N-(5-methylthiazol-yl)pentadecamide] to 3h [N-(5-methylthiazol-yl)stearamide] were synthesized, characterized and investigated for their gelation properties. Interestingly, out of three series of thiazole amides synthesized, two (1e–1h and 3e–3h) had displayed odd–even effect on gelation property with an increase in the methylene functional group of alkyl chain attached with thiazole moiety. The gelation–non-gelation of solvents was found to be more significant for the series of compounds 1e–1h, whereas a subtle effect was observed in the series of compounds 3e–3h. A single crystal study of non-gelator (2d) highlighted the crucial role of the methyl group and its position on the thiazole moiety in bringing about a change in supramolecular synthon from a robust cyclic N–H⋯N interaction to the combination of N–H⋯N and N–H⋯O interactions. Self-assembly of four molecules of 2d led to the formation of a zero-dimensional (0-D) hydrogen bonded network instead of a one-dimensional hydrogen bonded network observed in gelling compounds mediated by (methyl)C–H⋯N, C–H⋯O and van der Waals interaction. Various gelling agents (3e–3h) were used for the synthesis of nearly spherical silver and ZnO nanoparticles using a sol–gel method, through encapsulation and stabilization of nanoparticles in the gel network. Interestingly, the alkyl chain lengths of thiazole amides were found to affect the size of synthesized Ag and ZnO nanoparticles.
Introduction
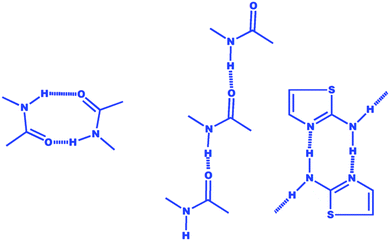
Small organic compounds having a molecular mass typically <3000, which are capable of arresting the flow of liquids (gel formation), are popularly known as low molecular mass organic gelators (LMOGs).1 LMOGs are attracting much attention due to the structural diversity of gelling compounds and their potential applications in drug release,2 nanotechnology,3 pollution control,4 oil-spill recovery,5 sensors,6 in preparing dye sensitized solar cells,7 tissue engineering,8 and many more.9 Generally, gelling molecules self-assemble into various recognizable shapes, such as fibres, filaments, tapes, ribbons, and tubes, which entangle among themselves to create a three-dimensional (3D) network capable of immobilizing the solvent molecules. Moreover, well-defined supramolecular assemblies of gelator fibres provide an opportunity to design and synthesize one-dimensional inorganic nanomaterials, which are otherwise hard to synthesize, by various physical and chemical methods. Therefore, one of the most explored applications of LMOGs is the template directed synthesis of inorganic nanomaterials of various shapes and sizes. Numerous gelling systems, such as cholesterol based, crown ether appended cholesterol compounds, diamines based, sugar based, are well known as structure-directing agents for charged silica and titanium compounds.10 It is well-accepted that gelators having charge or many H-bond sites would be successful in transcription of silica–titanium compounds (charged precursor) into various shapes and sizes. Unfortunately, no such hypothesis/working rule is available to transcript neutral metal or metal oxide successfully onto gelator fibres. Thus, it is required to explore various structural attributes, functional groups, critical balance of hydrophobicity–hydrophilicity of LMOGs required for designing template-directed synthesis of nanomaterials of various shapes and sizes. Moreover, tuning nanomaterial shapes and sizes by systematic variation in gelator structure is also desirable.Recently, we discovered a new series of LMOGs based on thiazole amide derivatives (1a–1d, 2a–2d and 3a–3d)11 (Scheme 1), which displayed the importance of position of a methyl group on a thiazole moiety in inducing gelation of solvent. For example, in the series of 5-methylthiazole amides (3b and 3d), gelation behaviour towards many solvents were observed, but gelation was completely absent in the series of 4-methylthiazole amides (2a–2d). Moreover, thiazole amide derivatives without methyl substitution displayed gelation property to a lesser extent; fewer number of solvents gellified, requiring a higher concentration of gelator for gelation, i.e. high critical gelator concentration (cgc). We proposed the crucial role of a methyl group in the formation of a weak hydrogen bond, namely, (methyl)C–H⋯N(thiazole), leading to a one dimensional hydrogen bonded network, recognized to be a pre-requisite for organogelation,12 based on single crystal X-ray study of gelator–non-gelator molecules and variable temperature 1H NMR. Our best efforts to grow crystals of non-gelator (2a–2d) failed, which deprived us of providing unequivocal proof of the proposed mechanism of organogelation based on (methyl)C–H⋯N(thiazole) and van der Waals interaction. Moreover, some of the fundamental questions about the mechanism of gelation need to be addressed such as (i) is it possible to induce gelation property to a non-gelator by systematic increase in the hydrocarbon chain/hydrophobicity?; (ii) what is the effect of an alkyl chain on the gelation behavior of the molecules in polar solvents or non-polar solvents in these series of compounds?; (iii) is the proposed weak hydrogen bond, such as (methyl)C–H⋯N, robust enough to induce gelation or non-gelation property to a molecule?; (iv) does the odd–even effect commonly observed in various series of supramolecular gels13 play a role in these series of molecules?; (v) how predictable is supramolecular synthon of amide in the presence of competitive cyclic N–H⋯N interaction in thiazole containing amides (Scheme 2)?; and (vi) is it possible to gain control over the shape and size of nanomaterials synthesized by a sol–gel method by a methodical change in the alkyl chain length of gelator molecules?
In the present study, we decided to systematically increase the alkyl chain length of gelling and non-gelling molecules having a methylene group (CH2) ranging from 8, 10–16 to see the interplay of a weak hydrogen bond (methyl)C–H⋯N or (thiazole)C–H⋯N, C–H⋯π, etc. along with van der Waals interaction in inducing gelation–non-gelation behaviour (Fig. 1). The efforts were directed towards growing more crystals of gelling–non-gelling compounds in the series to establish structure–property correlation. Fortunately, we are able to grow crystals of non-gelator (2d) along with gelator molecules (1c, 1d and 3c), which helped us to understand the role of weak H-bond interaction and alkyl chain interdigitation on gelation behavior. The potential applications of gelators 3a–3h as a template for the synthesis of silver and zinc oxide (ZnO) nanomaterial were explored. Template direct synthesis of ZnO nanoparticles was undertaken due to ZnO intriguing chemical, electrical, mechanical and optical properties and its potential application in solar cells, hydrogen-storage, gas sensors, liquid crystal displays, etc.14 Moreover, the properties and applications of the ZnO nanoparticles strongly depend upon their structures and morphologies.
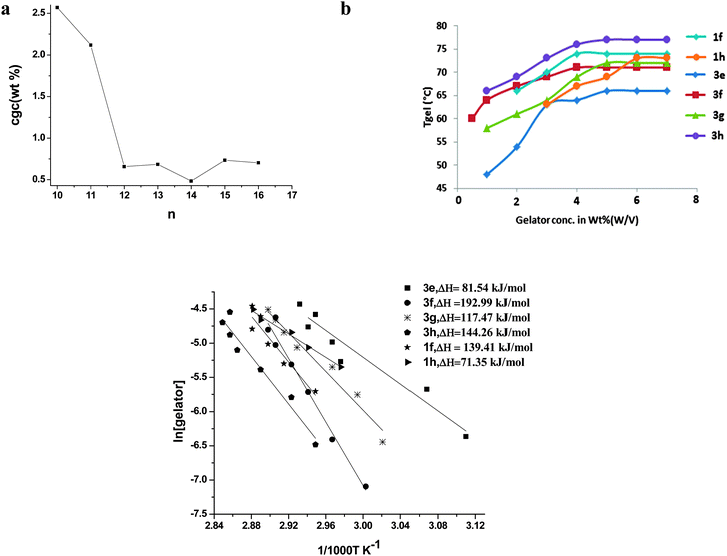 | ||
| Fig. 1 (a) Critical gelator concentration (wt%, w/v) of 3b–3h in methanol versus number of methylene groups (n); (b) Tgelversus concentration plot of gel in methanol; and (c) semilog plot of the mole fraction of the gelators against 1/1000 T (K−1), where ΔHm and Tgel are the enthalpy of melting and transition temperature of gel-to-sol, respectively (Tgel value of compounds 3b–3d is taken from ref. 11). | ||
Results and discussion
Gelation behaviour
The synthesis, characterization, and gelation behaviour of thiazole amides (1a–1d, 2a–2d and 3a–3d) were reported earlier (n = 8, 10–12).11 In the present study, gelation abilities of 1e–1h, 2e–2h and 3e–3h were examined in a variety of polar and non polar solvents (ESI,† Tables S1 and S2) by the systematic increase in the alkyl chain length (methylene functional group (n) = 13–16). Unsubstituted (1e–1h) and 5-methyl substituted (3e–3h) thiazole amides displayed excellent gelation properties towards various solvents such as methanol, ethanol, octadecane, etc. with low critical gelation concentration (cgc) (wt%, w/v). A significant enhancement and/or retardation of gelation property in the new series of compounds were observed with the increase in methylene groups, which corroborate the role of flexible alkyl chain in effecting organogelation behaviour significantly.15 Compounds 1a–1h displayed interesting gelation behaviour with the increase in methylene groups in alkyl chain, the compounds having an even number of methylene groups (n = even) were outstanding organogelators, whereas compounds having an odd number of methylene groups were weak gelators or non-gelator (odd–even effect). For example, compounds 1d (n = 12), 1f (n = 14) and 1h (n = 16) demonstrated organogelation, and compounds 1c (n = 11), 1e (n = 13) and 1g (n = 15) showed weak gelation or no-gelation at all (ESI,† Fig. S1). The effect of the odd–even number of methylene groups of alkyl chains on the gelation behaviour of these series of compounds is quite pronounced.Similarly, the series of gelators (3b–3h) with an odd number of methylene units (n = 11, 13, 15) in the alkyl tail required large amounts of gelling compound to exhibit gelation of solvent (larger cgc value) as compared with compounds having an even number of methylene units (n = 10, 12, 14, 16), suggesting the odd–even effect in this series of compounds, though less distinct than 1a–1h series of compounds. Moreover, the odd–even effect of alkyl chain length becomes more effective after reaching a critical chain length, i.e. n > 12 in this series of thiazole amides. A representative graph to show the effect of increase in number of methylene group ‘n’ in the aliphatic chain length of thiazole amides (3a–3h) on critical gelator concentration (cgc) (wt%, w/v) in methanol is shown in Fig. 1a.
Concentration-dependent gelation studies of all the gelators were carried out in ethanol. The dependence of Tgel on the concentration of gelator in ethanol is shown in Fig. 1b. Tgel increases rapidly with concentration up to a certain wt% and then shows independence towards the increase in concentration of gelator. Evidently, the increase in concentration of gelator enhances the gel-to-sol transition temperature by actively participating in the formation of gelator fibres or by improving intermolecular interaction up to a certain concentration. After reaching the critical concentration effect on the strength of gelator fibres, its interaction with gelling solvent, as represented by Tgel values, reaches a maximum value. The Tgel values of compounds 3e–3f before saturation demonstrated a gradual increase with the increase in the alkyl chain length, which supports the function of van der Waals interaction in enhancing the stability of the gel network. Furthermore, the gelators having an even number of methylene groups (3f and 3h) displayed higher value of gel–sol transition temperature than the gelators having an odd number of methylene groups (3e and 3g) in the alkyl chain as seen in Fig. 1b at 4% wt. A linear relationship was obtained when a semilog graph of the mole fraction of organogelators was plotted against 1/1000 Tgel (K−1) which agreed well with the Schroeder–van Laar equation (eqn (1), Fig. 1c).
| ln[gelator] = −(ΔHm/RTgel) + constant | (1) |
The gel-to-sol transition can be considered a first order transition assuming that the gel melts into an ideal solution and a known amount of gelator is involved in the transition.16 From the plots, the enthalpy ΔHm was calculated to be within the range of 71–192 kJ mol−1. Strikingly, enthalpy of melting of gel network (gel–sol transition) in the series 1e–1h also showed the odd–even effect. ΔHm of gel breakdown in the case of 3e and 3g (n = odd) was found to have lower values than 3f and 3h (n = even), suggesting the extra stability commanded by the gels of 3f and 3h.
Morphological studies of xerogel
Scanning electron microscopy (SEM) micrographs were recorded for visualizing xerogels morphologies derived from the gelators 1e, 1h, 3e, 3f, 3g and 3h (Fig. 2). Understandably, the solvent molecules were immobilized in a 3-D network of fibres in the gel state. Dried gelled networks of 1e and 1h displayed an augmented complexity of arrangement of gelator fibres with increase in methylene groups and better entanglement of gelator fibres. The progressive increase in the entanglement of gelator fibres becomes more pronounced in the case of gelators 3e–3h. A highly complex network of fibres was observed in the case of xerogel of 3h (at higher magnification) with highly distinct pores, which might have acted as a reaction chamber for synthesizing nanoparticles stabilized by gelator fibres as discussed later.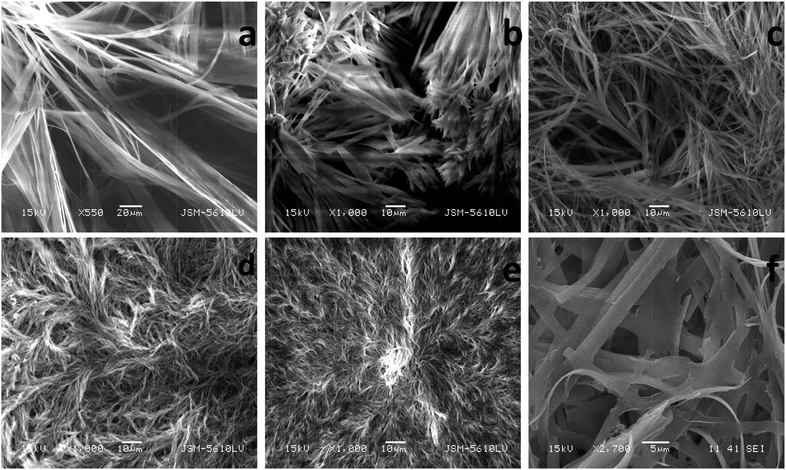 | ||
| Fig. 2 SEM images of xerogel of (a) 1e, (b) 1h (c) 3e (d) 3f (e) 3g and (f) 3h in methanol at 2 wt% (w/v). | ||
Single crystal X-ray studies
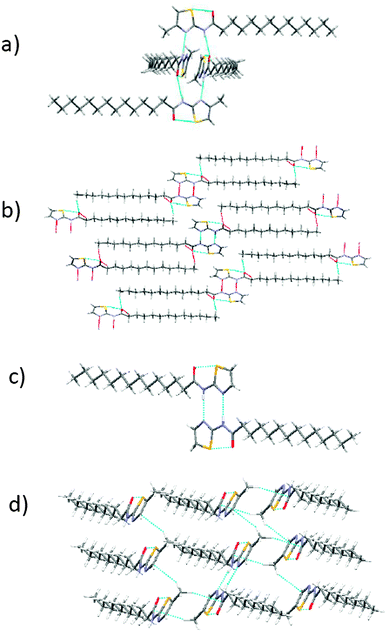
It is well-documented that gelation is a metastable state realized when crystallization fails.1 Therefore, gaining information about the packing of gelator molecules in the gel state is a daunting task. Nevertheless, some information about the packing of gelator molecules in xerogel (dried gel) could be obtained by comparing a simulated powder X-ray diffraction pattern of a single crystal X-ray structure of a gelator molecule (if any) with xerogel, a method developed by Weiss et al.17 Single crystal structure of gelators–non-gelators, if critically analyzed, may throw some light on the structural features and weak non-bonded interactions (supramolecular synthons) required to facilitate aggregation leading to a 3D network capable of arresting the flow of liquids (organic/aqueous).A single crystal of non-gelator 2d provided an opportunity to know the probable reason of its non-gelation behaviour towards all the solvents employed in the present study. A suitable crystal of 2d was obtained from methanol–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) by the slow evaporation method. 2d crystallized out in the monoclinic space group P21/c containing two molecules in the asymmetric unit. Four molecules of 2d were found to self-assemble together to form the 0-D hydrogen bonded network driven by N–H⋯N [N–H⋯N = 2.884 Å, ∠N–H⋯N = 173.84°] and N–H⋯O interaction [N–H⋯O = 2.937 Å, ∠N–H⋯O = 167.09°] (see Fig. 3a). A detailed analysis of the packing pattern of 2d showed weak van der Waals forces between the methyl group and the alkyl chain, which may be due to packing forces present in the crystalline phase. We ascertained that the presence of a 0-D network, the absence of alkyl chain interdigitation, and no additional hydrogen bonds such as C–H⋯N or C–H⋯O endowed on these sets of molecules (2a–2h), the incapability to immobilize any solvents. The single crystal study of 2d supported the proposed mechanism for gelation–non-gelation that the strategic position of the methyl functional group may sustain or obstruct alkyl–alkyl chain interdigitation.11 A suitable single crystal of gelator 1c was obtained from chloroform having a monoclinic (P21/c) space group. The asymmetric unit displayed a single molecule of 1c joined together with another molecule through cyclic hydrogen bond N–H⋯N [N–H⋯N = 2.942 Å, ∠N–H⋯N = 173.21°] leading to the formation of the 0-D network. The 0-D network was extended to form the 3-D network through multiple C–H⋯O interactions (Fig. 3b). A crystal of 1d was obtained from chloroform by the slow evaporation method. 1d crystallized out in the monoclinic space group P21/c and showed a robust cyclic (amide)N–H⋯N(thiazole) [N–H⋯N = 2.932 Å, ∠N–H⋯N = 174.64°] supramolecular synthon leading to a 0-D hydrogen bonded network along with O⋯S intramolecular interaction (Fig. 3c). 3c crystallized out in a space group triclinic P
20 v/v) by the slow evaporation method. 2d crystallized out in the monoclinic space group P21/c containing two molecules in the asymmetric unit. Four molecules of 2d were found to self-assemble together to form the 0-D hydrogen bonded network driven by N–H⋯N [N–H⋯N = 2.884 Å, ∠N–H⋯N = 173.84°] and N–H⋯O interaction [N–H⋯O = 2.937 Å, ∠N–H⋯O = 167.09°] (see Fig. 3a). A detailed analysis of the packing pattern of 2d showed weak van der Waals forces between the methyl group and the alkyl chain, which may be due to packing forces present in the crystalline phase. We ascertained that the presence of a 0-D network, the absence of alkyl chain interdigitation, and no additional hydrogen bonds such as C–H⋯N or C–H⋯O endowed on these sets of molecules (2a–2h), the incapability to immobilize any solvents. The single crystal study of 2d supported the proposed mechanism for gelation–non-gelation that the strategic position of the methyl functional group may sustain or obstruct alkyl–alkyl chain interdigitation.11 A suitable single crystal of gelator 1c was obtained from chloroform having a monoclinic (P21/c) space group. The asymmetric unit displayed a single molecule of 1c joined together with another molecule through cyclic hydrogen bond N–H⋯N [N–H⋯N = 2.942 Å, ∠N–H⋯N = 173.21°] leading to the formation of the 0-D network. The 0-D network was extended to form the 3-D network through multiple C–H⋯O interactions (Fig. 3b). A crystal of 1d was obtained from chloroform by the slow evaporation method. 1d crystallized out in the monoclinic space group P21/c and showed a robust cyclic (amide)N–H⋯N(thiazole) [N–H⋯N = 2.932 Å, ∠N–H⋯N = 174.64°] supramolecular synthon leading to a 0-D hydrogen bonded network along with O⋯S intramolecular interaction (Fig. 3c). 3c crystallized out in a space group triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . H-bonding pattern in the crystal seems to be governed by cyclic (amide)N–H⋯N(thiazole) synthons [N–H⋯N = 2.941 Å, ∠N–H⋯N = 173.18°] along with the intramolecular bond between carbonyl oxygen and thiazole sulphur atom, leading to a zero-dimensional (0-D) hydrogen bonded network. A critical examination of the crystal structures demonstrated that a weaker hydrogen bond (methyl)C–H⋯C(thiazole) (distance between C–H⋯π = 3.224 Å, ∠C–H⋯C = 153.21°) and hydrogen bond (methyl)C–H⋯C(carbonyl carbon) (distance between C–H⋯C = 2.879 Å, ∠C–H⋯C = 160.67°) leads to a two-dimensional (2-D) hydrogen bonded network (Fig. 3d).
. H-bonding pattern in the crystal seems to be governed by cyclic (amide)N–H⋯N(thiazole) synthons [N–H⋯N = 2.941 Å, ∠N–H⋯N = 173.18°] along with the intramolecular bond between carbonyl oxygen and thiazole sulphur atom, leading to a zero-dimensional (0-D) hydrogen bonded network. A critical examination of the crystal structures demonstrated that a weaker hydrogen bond (methyl)C–H⋯C(thiazole) (distance between C–H⋯π = 3.224 Å, ∠C–H⋯C = 153.21°) and hydrogen bond (methyl)C–H⋯C(carbonyl carbon) (distance between C–H⋯C = 2.879 Å, ∠C–H⋯C = 160.67°) leads to a two-dimensional (2-D) hydrogen bonded network (Fig. 3d).
One of the major challenges in these series of molecules is to understand the cause of gelation–non-gelation behaviour, due to odd–even number of methylene functional groups. Similar behaviour by urea functional groups containing gelators is well known and such behaviour was assigned to a favourable anti or syn hydrogen bonded network with the change in alkyl chain length.13c However, no direct proof is available in amide based gelling systems to show such interactions.13d Interestingly, all the gelling–non-gelling molecules in these series showed robust N–H⋯N hydrogen bonding between thiazole nitrogen and amide nitrogen instead of an amide–amide functional group interaction (Scheme 2). Our observations on the supramolecular assembly of amide containing thiazole compounds are well supported by the observation made by Aakeröy et al.18 in the seminal paper on amide functionality as robust synthon and the prospect of amide–amide interactions (0-D or ladder type) in the presence of other probable hydrogen bonded supramolecular synthons (amide–amide interaction is less probable in the presence of strong N–H⋯N type cyclic H-bond). Interestingly, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯S (intramolecular) forces found in all the crystal structures of thiazole amide were reported in the present study as well as in our earlier work11 irrespective of the presence–absence or position of the methyl functional group. The unusual non-bonded interaction, (carbonyl)C
O⋯S (intramolecular) forces found in all the crystal structures of thiazole amide were reported in the present study as well as in our earlier work11 irrespective of the presence–absence or position of the methyl functional group. The unusual non-bonded interaction, (carbonyl)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯S(thiazole), appeared to force the long alkyl chain to have almost linear orientation with respect to the thiazole moiety.19 Logically, the linearity of alkyl chains would favour interdigitation between alkyl chains along with the opportunity of formation of weaker H-bonds such as C–H⋯O, C–H⋯N, etc. On the other hand, the proximity of a methyl group to alkyl chains would disturb the overall packing pattern and probably lead to the 0-D dimensional hydrogen bonded network as observed in the structure of non-gelator 2d. Single crystal structures of 1b (even, n = 10)11 and 1c (odd, n = 11) highlighted additional C–H⋯O interaction in 1c leading to 2-D hydrogen bonded network in comparison to 1b, which is a 0-D network.
O⋯S(thiazole), appeared to force the long alkyl chain to have almost linear orientation with respect to the thiazole moiety.19 Logically, the linearity of alkyl chains would favour interdigitation between alkyl chains along with the opportunity of formation of weaker H-bonds such as C–H⋯O, C–H⋯N, etc. On the other hand, the proximity of a methyl group to alkyl chains would disturb the overall packing pattern and probably lead to the 0-D dimensional hydrogen bonded network as observed in the structure of non-gelator 2d. Single crystal structures of 1b (even, n = 10)11 and 1c (odd, n = 11) highlighted additional C–H⋯O interaction in 1c leading to 2-D hydrogen bonded network in comparison to 1b, which is a 0-D network.
A subtle odd–even effect was observed in the series of compounds 3d–3h which showed increased or decreased cgc values with one additional methylene group, which can be attributed to a suitable positioning of alkyl chains over one another and increasing interdigitation (n = even) along with additional C–H⋯O hydrogen bond. The variable temperature 1H-NMR of gelling (1d and 3d) and non-gelling compounds (2d) supported the retention of a H-bonding pattern (no significant chemical shift of protons) in gel and sol phases, whereas non-gelling compounds showed a shift of thiazole proton, a mark of its participation in the H-bond. The following results corroborated the IR studies carried out in solid, gel and solution phases of gelling compounds.11
A pronounced effect of an increase in alkyl chain length could be observed in the series of compounds 1a–1h, which displayed complete presence (n = even) or absence (n = odd) of gelation property. Structures reported by us displayed an additional (methyl)C–H⋯O bond along with predominant C–H⋯N interaction with an increase in alkyl chain length from 3c (n = 10) to 3e (n = 12). We proposed an interplay of weak interaction such as C–H⋯O, C–H⋯N as the driving force for gelation in these series of compounds along with robust supramolecular synthons N–H⋯N and N–H⋯O interaction. A favourable presence of alkyl–alkyl chain interdigitation enhances the gelation properties and its absence in the supramolecular assembly of thiazole amides (2a–2e) loses its capability to immobilize any of the solvents used in the present study. The powder X-ray diffraction (PXRD) studies of xerogel of gelators–non-gelators are frequently being carried out to get an insight into the packing of gelator fibres. PXRD of xerogel of gelling and non-gelling compounds with an even–odd number of methylene functional groups was recorded. 3b (n = 12, even), 1d and 3d (n = 14, even) showed a periodic high intensity peak position in the lower 2 theta angle in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3, respectively, suggesting lamellar packing such as 21.6034, 11.0243, 7.5231 for 1d; 22.6578, 11.8392, 7.8609 for 3b; and 21.1379, 11.6241, 7.0921 for 3d (ESI,† Fig. S2). However, in the case of 3c and 1c (n = 13, odd) such periodic peaks were not observed. Based on these observations, we propose that alkyl–alkyl chain interdigitation plays a significant role in producing an odd–even effect in the series of compounds 1a–1h and 3a–3h.
1/3, respectively, suggesting lamellar packing such as 21.6034, 11.0243, 7.5231 for 1d; 22.6578, 11.8392, 7.8609 for 3b; and 21.1379, 11.6241, 7.0921 for 3d (ESI,† Fig. S2). However, in the case of 3c and 1c (n = 13, odd) such periodic peaks were not observed. Based on these observations, we propose that alkyl–alkyl chain interdigitation plays a significant role in producing an odd–even effect in the series of compounds 1a–1h and 3a–3h.
Template directed synthesis of Ag and ZnO nanoparticles
ZnO nanoparticles-loaded gelator samples were prepared by a sol–gel method. Thermogravimetric analyses (TGA) of the samples were carried out to verify the removal of the gelator (ESI,† Fig. S3) from the synthesized ZnO nanoparticles. TGA analysis of the synthesized samples displayed a weight loss of 74.4% corresponding to the complete removal of the gelator molecules at 350 °C. Powder X-ray diffractograms were recorded to provide conclusive evidence for the formation silver and ZnO nanoparticles (ESI,† Fig. S4). The peaks were observed at 2 theta angles of 31, 39 and 45 corresponding to the (101), (111) and (200) crystalline planes of Ag, respectively.20 Similarly, the peaks were observed at diffraction angles 31, 36, 47, 56, 62 and 67 corresponding to the (100), (101), (102), (110), (103) and (112) crystalline planes of ZnO, respectively.21TEM images were used to characterize the external morphology of the synthesized Ag and ZnO nanoparticles. TEM imaging was carried out on Ag nanoparticles embedded in gel fibres, which act as stabilizing and capping agents (Fig. 4). Nearly spherical nanoparticles of Ag were observed in the TEM images, suggesting successful encapsulation of silver nanoparticles in a gel network stabilized by gelator fibres of 3b–3h. The range of diameters of silver nanoparticles, determined using TEM images of silver embedded in the gelled medium of 3b, 3e, 3f and 3h, were found to increase with increase in the alkyl chain length (Table 1). A progressive increase in the nanoparticles size with the increase in alkyl chain length suggested the probable increase in the void between 3-D networks of gelator fibres. ZnO nanoparticles were subjected to TEM analysis after complete removal of gelator network (calcination) (Fig. 5). The range of diameters for the series of ZnO nanoparticles based on TEM images of 3b, 3e, 3f and 3h are shown in Table 1.
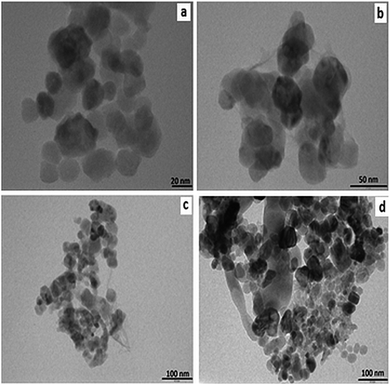 | ||
| Fig. 4 TEM images of silver nanoparticles formed within the gel network of (a) 3b (b) 3e (c) 3f (d) 3h. | ||
| Range of diameters (nm) (Ag nanoparticles) | Range of diameters (nm) (ZnO nanoparticles) | |
|---|---|---|
| 3b (n = 10) | 5–20 | 10–20 |
| 3e (n = 13) | 18–30 | 20–30 |
| 3f (n = 14) | 25–37 | 24–40 |
| 3h (n = 16) | 30–50 | 28–52 |
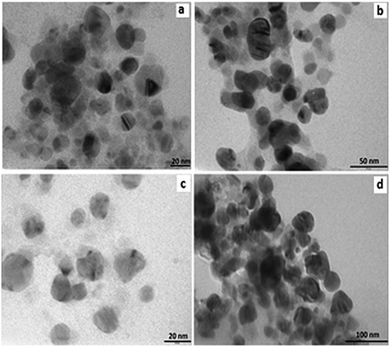 | ||
| Fig. 5 TEM images of ZnO nanoparticles formed within the gel network of (a) 3b (b) 3e (c) 3f (d) 3h. | ||
The synthesized ZnO nanoparticles also displayed a gradual increase in size with one additional methylene functional group change, which corroborates the hypothesis that nanoparticles were synthesized in the well-defined void created by gelator fibres.
A probable mechanism of nanoparticle synthesis is proposed in Scheme 3 based on single crystal studies of gelator–non-gelator and various physico-chemical analyses of thiazole based amide, and synthesized nanoparticles.
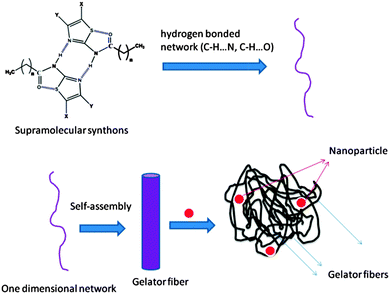 | ||
| Scheme 3 Proposed mechanism of template directed synthesis of nanoparticles in thiazole amide based gelator. | ||
Conclusion
In conclusion, we have synthesized and characterized new series of compounds 1e–1h, 2e–2h and 3e–3h by systematically changing the alkyl chain length. A significant odd–even effect was observed in the series of compounds 1a–1h and 3a–3h beyond a certain critical chain length, i.e. n = 8 for 3 (3a) and n = 11 for 1 (1c). The odd–even effect of gelling molecules was attributed to the interplay between numerous hydrogen bonds such as C–H⋯O and C–H⋯N, as well as van der Waals interaction between alkyl chains (interdigitation). The series of unsubstituted thiazole amide seems to induce gelation of organic solvents through a hydrogen bonded network supported by weak van der Waals interaction. The alkyl–alkyl chain interdigitation is conducive in the case of even numbers of methylene groups, which supported gelation, whereas awkward packing of the alkyl chain in the case of odd numbers of methylene groups of the series (1a–1h) leads to complete non-gelation behaviour. A less prominent odd–even effect of methylene groups on organogelation properties of 3a–3h may be attributed to the synergistic effect of (methyl)C–H⋯N(weak) and N–H⋯N(strong) H-bonds capable of formation of a 3-D network sufficient to immobilize a solvent in the presence and/or absence of alkyl–alkyl interdigitation. A low cgc value and better efficiency of organogelators 3b–3h, when ‘n’ is even, may be assigned to the extra stability provided by a favourable alkyl–alkyl chain interaction. The present study establishes the weak H-bond (methyl)C–H⋯N as a crucial interaction leading to a 1-D network in 5-methylthiazole amide derivatives, whereas 4-methyl thiazole amide derivatives formed a 0-D network in the absence of such interactions. Our observations match with the known fact that a 1D network favours organogelation, whereas a 2D/3D network leads to weak gel or no gel at all.12 Nearly spherical Ag and ZnO nanoparticles synthesis was successfully carried out in gelator assembly of the series 3b–3h. In the absence of anisotropic nanoparticles such as nanorods, ribbons, and tubes, which is frequently observed in the case of template directed synthesis using LMOGs, we believe that the nanoparticle synthesis takes place in the core of the supramolecular gel assembly in agreement with the earlier known cases of in situ silver nanoparticles synthesis in supramolecular gels.22 The gelator fibres play a role of encapsulation and stabilization of synthesized nanoparticles in the gel phase. The sizes of nanoparticles of Ag and ZnO were found to be dependent on the alkyl chain length of thiazole amides (3b–3h), signifying variation in the complexity of the gelator network and tunability of pore size between fibres, due to systematic increase in the van der Waals interaction and interdigitation of alkyl chains.Experimental section
Materials
2-Aminothiazole (97%), 2-amino-5-methylthiazole (98%), 2-amino-4-methylthiazole (98%), octadecanoic acid (97%), heptadecanoic acid (>98%), hexadecanoic acid (98%), pentadecanoic acid (99%) (all from Aldrich) were used as received. The other chemicals were of the highest commercial grade available and were used without further purification. The solvents used for the preparation of gels were reagent grade. All solvents used in the synthesis were purified, dried and distilled as required.All thiazole based amide derivatives were synthesized by reacting the acid chloride of various aliphatic acids (pentane carboxylic acid to octadecane carboxylic acid) with thiazole derivatives (Fig. 1) using a modified synthetic procedure.23 Detailed synthesis, characterizations and gelation properties of compounds 1a–d, 2a–d and 3a–d were reported by us.11 All new compounds synthesized in the present study, 1e–1h, 2e–2h and 3e–3h, were characterized by IR, 1H-NMR, and MS analysis.
General synthesis methodology
Oxalyl chloride (2 ml, 20 mmol) was added slowly to a stirred solution of monocarboxylic acids (2 mmol) in dry dichloromethane (10 ml) under a nitrogen atmosphere, and stirring was continued under a nitrogen atmosphere for 12 h. Excess oxalyl chloride and solvent were removed by distillation under reduced pressure. The acid chloride obtained was dissolved in dry dichloromethane (10 ml) and added to the aminothiazole (2 mmol) in triethylamine (0.3 ml, 2.15 mmol). The mixture was stirred under a nitrogen atmosphere overnight. The reaction mixture was then added to dilute hydrochloric acid (5%), and extracted with chloroform. The product residue after removing chloroform was purified by repeated crystallization from ethyl acetate–pet. ether mixture.1e: Yield 85%, m.p. 105 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 12.165 (s, 1H, NH), 7.457 (d, 1H; CH), 7.023 (d, 1H; CH), 2.590–2.553 (t, 2H; CH2), 1.827–1.752 (m, 2H, CH2), 1.424–1.264 (m, 22H, CH2), 0.910 (t, 3H; CH3). MS (EI): m/z 324.53 [M]+. FTIR (KBr): 3171, 2916, 2849, 1688, 1577, 1468, 1321, 1279, 1166, 1065, 959, 874, 805, 778, 624, 520 cm−1.
1f: Yield 80%, m.p. 121 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 12.053 (s, 1H, NH), 7.458 (d, 1H; CH), 7.026 (d, 1H; CH), 2.585–2.547 (t, 2H; CH2), 1.888–1.771 (m, 2H, CH2), 1.424–1.265 (m, 24H, CH2), 0.911–0.876 (t, 3H; CH3). MS (EI): m/z 338.31 [M]+. FTIR (KBr): 3174, 2917, 2849, 1686, 1581, 1468, 1380, 1322, 1288, 1110, 1067, 959, 873, 777, 718, 625, 520 cm−1.
1g: Yield 83%, m.p. 117 °C, 1H NMR (400 MHz CDCl3, TMS): δ 11.9855 (s, 1H, NH), 7.462 (d, 1H; CH), 7.032 (d, 1H; CH), 2.590–2.365 (t, 2H; CH2), 1.827–1.752 (m, 2H, CH2), 1.437–1.264 (m, 26H, CH2), 0.911–0.877 (t, 3H; CH3). MS (EI): m/z 351.84 [M]+. FTIR (KBr): 3170, 2917, 2849, 1685, 1576, 1469, 1379, 1321, 1272, 1168, 1064, 959, 875, 778, 718, 624, 520 cm−1.
1h: Yield 79%, m.p. 102 °C, 1H-NMR (400 MHz, CDCl3, TMS): δ 11.865 (s, 1H, NH), 7.458 (d, 1H; CH), 7.024 (d, 1H; CH), 2.581–2.544 (t, 2H; CH2), 1.828–1.753 (m, 2H, CH2), 1.430–1.265 (m, 28H, CH2), 0.912–0.878 (t, 3H; CH3). MS (EI): m/z 366.18 [M]+. FTIR (KBr): 3176, 2924, 2851, 1683, 1580, 1465, 1379, 1323, 1275, 1169, 1065, 962, 872, 774, 718, 623, 520 cm−1.
2e: Yield 72%, m.p. 100 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 12.165 (s, 1H, NH), 6.506 (s, 1H; CH), 2.507–2.478 (t, 2H; CH2), 2.346 (s, 3H; CH3), 1.768–1.712 (m, 2H; CH2), 1.330–1.266 (m, 22H; CH2), 0.911–0.880 (t, 3H; CH3). MS (EI): m/z 338.39 [M]+. FTIR (KBr): 3358, 3164, 2928, 2854, 1672, 1553, 1469, 1314, 1235, 1115, 1079, 974, 772, 564 cm−1.
2f: Yield 70%, m.p. 83 °C, 1H NMR (400 MHz, CDCl3, TMS); δ 13.360 (s, 1H, NH), 6.652 (s, 1H; CH) 2.698–2.660 (t, 2H; CH2), 2.511 (s, 3H; CH3), 1.801–1.765 (m, 2H; CH2), 1.377–1.311 (m, 24H; CH2), 0.908–0.874 (t, 3H; CH3). MS (EI): m/z 352.29 [M]+. FTIR (KBr): 3348, 3152, 2914, 2849, 1670, 1552, 1461, 1317, 1233, 1117, 1085, 976, 778, 561 cm−1.
2g: Yield 74%, m.p. 89 °C, 1H NMR (400 MHz, CDCl3, TMS); δ 13.345 (s, 1H, NH), 6.526 (s, 1H; CH), 2.557–2.422 (t, 2H; CH2), 2.262 (s, 3H; CH3), 1.777–1.642 (m, 2H; CH2), 1.331–1.208 (m, 26H; CH2), 0.911–0.877 (t, 3H; CH3). MS (EI): m/z 366.40 [M]+. FTIR (KBr): 3359, 3160, 2923, 2859, 1675, 1559, 1463, 1329, 1239, 1121, 1090, 982, 770, 569 cm−1.
2h: Yield 71%, m.p. 96 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 13.178 (s, 1H, NH), 6.559 (s, 1H; CH), 2.501–2.463 (t, 2H; CH2), 2.238 (s, 3H; CH3), 1.756–1.720 (m, 2H; CH2), 1.331–1.267 (m, 28H; CH2), 0.912–0.896 (t, 3H; CH3). MS (EI): m/z 380.20 [M]+. FTIR (KBr): 3355, 3154, 2918, 2851, 1678, 1552, 1468, 1311, 1233, 1111, 1089, 976, 777, 560 cm−1.
3e: Yield 78%, m.p. 105 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 12.191 (s, 1H, NH), 7.085 (s, 1H; CH), 2.546–2.526 (t, 2H; CH2), 2.451 (s, 3H; CH3), 1.772–1.767 (m, 2H; CH2), 1.278–1.266 (m, 22H; CH2), 0.912–0.878 (t, 3H; CH3). MS (EI): m/z 338.19 [M]+. FTIR (KBr): 3179, 3060, 2912, 2847, 1682, 1589, 1466, 1418, 1381, 1303, 1279, 1181, 958, 719, 526 cm−1.
3f: Yield 82%, m.p. 121 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 11.986 (s, 1H, NH), 7.062 (s, 1H; CH), 2.532–2.494 (t, 2H; CH2), 2.431 (s, 3H; CH3), 1.808–1.739 (m, 2H; CH2), 1.267–1.224 (m, 24H; CH2), 0.913–0.897 (t, 3H; CH3). MS (EI): m/z 352.20 [M]+. FTIR (KBr): 3427, 3180, 3080, 2919, 2850, 1697, 1585, 1462, 1409, 1381, 1311, 1280, 1111, 953, 832, 723, 527 cm−1.
3g: Yield 80%, m.p. 111 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 12.005 (s, 1H, NH), 7.075 (s, 1H; CH) 2.531–2.494 (t, 2H; CH2), 2.437 (s, 3H; CH3), 1.806–1.752 (m, 2H; CH2), 1.398–1.266 (m, 26H; CH2), 0.912–0.893 (t, 3H; CH3). MS (EI): m/z 366.63 [M]+. FTIR (KBr): 3432, 3181, 2919, 2850, 2274, 1697, 1588, 1472, 1380, 1312, 1166, 1112, 1067, 962, 831, 782, 622 cm−1.
3h: Yield 79%, m.p. 108 °C, 1H NMR (400 MHz, CDCl3, TMS): δ 12.173 (s, 1H, NH), 7.088 (s, 1H; CH), 2.533–2.515 (t, 2H; CH2), 2.497 (s, 3H; CH3), 1.787–1.752 (m, 2H; CH2), 1.331–1.266 (m, 28H; CH2), 0.912–0.878 (t, 3H; CH3). MS (EI): m/z 380.35 [M]+. FTIR (KBr): 3428, 3179, 2919, 2850, 1696, 1583, 1465, 1411, 1380, 1311, 1278, 1168, 1108, 793, 722 cm−1.
Characterization
FTIR spectra were recorded on a Perkin Elmer – RX FTIR instrument. Solid samples were recorded as an intimate mixture with powdered KBr. The 1H-NMR spectra were measured using a Bruker AVANCE, 400 MHZ with TMS as internal standard. Morphologies of all reported xerogels (dried gels) were investigated using scanning electron microscopy (SEM) (JEOL JSM5610 LV microscope). For SEM study, the hot sample gel liquid was placed over the SEM sample holder and allowed to form the gel. Then, the sample was subjected to dryness under normal room temperature and pressure and coated with carbon (1e, 1h, 3e–3g) and gold sputtering (3h). The morphologies of synthesized Ag and ZnO nanoparticles were investigated using transmission electron microscopy (TEM) (Philips CM 200) in the working voltage of 20–200 kV. TEM studies were carried out by placing a small amount of the corresponding compound dispersed in ethanol–water on carbon-coated copper grids and dried by slow evaporation. The range of diameter of the synthesized nanoparticles was determined by measuring the diameters of <100 nanoparticles (from smallest to largest) using TEM images. Powder diffraction patterns of neat xerogel 1e (methanol, slowly evaporated) and xerogel loaded with silver nanoparticles were recorded on XPERT Philips (CuKα radiation). The silver nano-particles loaded on gelator fibres was subjected to powder diffraction studies using Bruker D8 (CuKα radiation) diffractometer. Single crystal X-ray study of compounds 1c, 1d, 2d and 3c was carried out on single crystal X-ray diffractometer (Xcalibur, EOS, Gemini diffractometer). All structures were analysed using Olex1.2 software24 and refined using ShelXL refinement package.25Gelation test
A weighted amount of potential gelator (10 mg) and a measured volume (1 ml) of selected pure organic solvent–water were placed into a capped test tube (outer diameter 10 mm and length 75 mm), and the system was heated in an oil or water bath, until the dissolution of solid materials. The solution was cooled to room temperature and finally, the test tube was turned upside down to observe if the solution inside could still flow or not (test-tube inversion method). No flow of the solvent was designated as gelation of the solvent. Gel-to-sol transition temperatures (Tgel) were determined using a conventional “dropping ball” method. In this test, a small glass ball (63 mg) was carefully placed on the top of the gel to be tested, which was present in a test tube. The tube was slowly heated in a thermostated oil bath until the ball fell to the bottom of the test tube. The temperature at which the ball reaches the bottom of the test tube is taken as Tgel of that system.Synthesis of silver nanoparticle
To load the organogels with AgNO3 for the synthesis of silver nanostructure, AgNO3 (2 mg) and the gelator (2 wt%) were dissolved in ethanol (2 ml) by heating. The solution was allowed to stand to form a colourless gel. The gel was irradiated with a mercury vapour UV lamp. On irradiation, there was a visually observable change to the gel: the colour changed rapidly from colourless to pale pink within 15 minutes of irradiation and it changed to reddish brown over a period of an hour.Synthesis of ZnO nanoparticles
To prepare ZnO particles, 35 mg zinc acetate was mixed with 10 mg KOH in 1 ml ethanol. This mixture was added to a solution containing 20 mg gelator in 1.0 ml ethanol. The mixture was heated and finally allowed to cool. A white gel thus obtained was allowed to stand for a day at ambient temperature and then dried in a vacuum. In order to remove the template, the sample was heated to 400 °C for 4 h.26Acknowledgements
P.Y. is thankful to UGC, New Delhi, for research fellowship (SMS/F.4-1/2006/XI Plan-BSR) and A.B. would like to acknowledge UGC, New Delhi, for financial support [MRP F. No. 37-379/2009 (SR)]. The authors would like to acknowledge the DST-PURSE programme for funding single crystal X-ray diffractometer facility at the Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat (India).Notes and references
- (a) P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3160 CrossRef CAS PubMed; (b) L. A. Estroff and A. D. Hamilton, Chem. Rev., 2004, 104, 1201–1217 CrossRef CAS PubMed; (c) J. A. Foster, R. M. Edkins, G. J. Cameron, N. Colgin, K. Fucke, S. Ridgeway, A. G. Crawford, T. B. Marder, A. Beeby, S. L. Cobb and J. W. Steed, Chem. – Eur. J., 2014, 20, 279–291 CrossRef CAS PubMed; (d) J. W. Steed, Chem. Soc. Rev., 2010, 39, 3686–3699 RSC; (e) X. Yu, L. Chen, M. Zhang and T. Yi, Chem. Soc. Rev., 2014, 43, 5346–5371 RSC.
- (a) K. J. Skilling, F. Citossi, T. D. Bradshaw, M. Ashford, B. Kellam and M. Marlow, Soft Matter, 2014, 10, 237–256 RSC; (b) M. Rodrigues, A. C. Calpena, D. B. Amabilino, M. L. Garduno-Ramirez and L. Perez-Garcia, J. Mater. Chem. B, 2014, 2, 5419–5429 RSC; (c) A. Y. Y. Tam and V. W. W. Yam, Chem. Soc. Rev., 2013, 42, 1540–1567 RSC; (d) L. Q. Chen, J. C. Wu, L. H. Yuwen, T. M. Shu, M. Xu, M. M. Zhang and T. Yi, Langmuir, 2009, 25, 8434–8438 CrossRef CAS PubMed.
- (a) S. Bhattacharya, A. Srivastava and A. Pal, Angew. Chem., Int. Ed., 2006, 45, 2934–2937 CrossRef CAS PubMed; (b) J. Nanda, B. Adhikari, S. Basak and A. Banerjee, J. Phys. Chem. B, 2012, 116, 12235–12244 CrossRef CAS PubMed; (c) S. K. Samanta, A. Pal, S. Bhattacharya and C. N. R. Rao, J. Mater. Chem., 2010, 20, 6881–6890 RSC; (d) B. Adhikari, J. Nanda and A. Banerjee, Chem. – Eur. J., 2011, 17, 11488–11496 CrossRef CAS PubMed; (e) S. K. Samanta, K. S. Subrahmanyam, S. Bhattacharya and C. N. R. Rao, Chem. – Eur. J., 2012, 18, 2890–2901 CrossRef CAS PubMed.
- (a) S. Kiyonaka, K. Sugiyasu, S. Shinkai and I. Hamachi, J. Am. Chem. Soc., 2002, 124, 10954–10955 CrossRef CAS PubMed; (b) K. Oishi and Y. Maehata, Chemosphere, 2013, 91, 302–306 CrossRef CAS PubMed; (c) S. R. Shirsath, A. P. Patil, R. Patil, J. B. Naik, P. R. Gogate and S. H. Sonawane, Ultrason. Sonochem., 2013, 20, 914–923 CrossRef CAS PubMed.
- (a) S. Bhattacharjya and Y. Krishnan-Ghosh, Chem. Commun., 2001, 185–186 RSC; (b) D. R. Trivedi, A. Ballabh and P. Dastidar, Chem. Mater., 2003, 15, 3971–3973 CrossRef CAS; (c) S. R. Jadhav, P. K. Vemula, R. Kumar, S. R. Raghavan and G. John, Angew. Chem., Int. Ed., 2010, 49, 7695–7698 CrossRef CAS PubMed; (d) S. Basak, J. Nanda and A. Banerjee, J. Mater. Chem., 2012, 22, 11658–11664 RSC; (e) A. Prathap and K. M. Sureshan, Chem. Commun., 2012, 48, 5250–5252 RSC.
- (a) I. Yoshimura, Y. Miyahara, N. Kasagi, H. Yamane, A. Ojida and I. Hamachi, J. Am. Chem. Soc., 2004, 126, 12204–12205 CrossRef CAS PubMed; (b) P. D. Wadhavane, M. A. Izquierdo, F. A. Galindo, M. I. Burguete and S. V. Luis, Soft Matter, 2012, 8, 4373–4381 RSC.
- (a) W. Kubo, T. Kitamura, K. Hanabusa, Y. Wada and S. Yanagida, Chem. Commun., 2002, 374–375 RSC; (b) H.-Y. Chen, L. Lin, X.-Y. Yu, K.-Q. Qiu, X.-Y. Lu, D.-B. Kuang and C.-Y. Su, Electrochim. Acta, 2013, 92, 117–123 CrossRef CAS PubMed; (c) Y. Rong, X. Li, G. Liu, H. Wang, Z. Ku, M. Xu, L. Liu, M. Hu, Y. Yang, M. Zhang, T. Liu and H. Han, J. Power Sources, 2013, 235, 243–250 CrossRef CAS PubMed.
- (a) C. Fan, L. Liao, C. Zhang and L. Liu, J. Mater. Chem. B, 2013, 1, 4251–4258 RSC; (b) S. Pok, J. D. Myers, S. V. Madihally and J. G. Jacot, Acta Biomater., 2013, 9, 5630–5642 CrossRef CAS PubMed.
- (a) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC; (b) Y. G. Li and M. H. Liu, Chem. Commun., 2008, 5571–5573 RSC; (c) A. Motulsky, M. Lafleur, A. C. Couffin-Hoarau, D. Hoarau, F. Boury, J. P. Benoit and J. C. Leroux, Biomaterials, 2005, 26, 6242–6253 CrossRef CAS PubMed; (d) J. Raeburn, A. Z. Cardoso and D. J. Adams, Chem. Soc. Rev., 2013, 42, 5143–5156 RSC; (e) X. H. Cao, A. P. Gao, H. T. Lv, Y. Wu, X. X. Wang and Y. Fan, Org. Biomol. Chem., 2013, 11, 7931–7937 RSC.
- (a) K. J. C. Van Bommel, A. Frigerri and S. Shinkai, Angew. Chem., Int. Ed., 2003, 42, 980–999 CrossRef CAS PubMed; (b) M. Llusar and C. Sanchez, Chem. Mater., 2008, 20, 782–820 CrossRef CAS.
- P. Yadav, D. Kaur, V. K. Gupta, Rajnikant and A. Ballabh, RSC Adv., 2013, 3, 8417–8421 CAS.
- P. Dastidar, Chem. Soc. Rev., 2008, 37, 2699–2715 RSC.
- (a) K. Aoki, M. Kudo and N. Tamaoki, Org. Lett., 2004, 6, 4009–4012 CrossRef CAS PubMed; (b) N. Fujita, Y. Sakamoto, M. Shirakawa, M. Ojima, A. Fujii, M. Ozaki and S. Shinkai, J. Am. Chem. Soc., 2007, 129, 4134–4135 CrossRef CAS PubMed; (c) M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Commun., 2008, 2644–2646 RSC; (d) M. Suzuki, M. Nanbu, M. Yumoto, H. Shirai and K. Hanabusa, New J. Chem., 2005, 29, 1439–1444 RSC.
- (a) Z. Fan and J. G. Lu, J. Nanosci. Nanotechnol., 2005, 5, 1561–1573 CrossRef CAS PubMed; (b) Q. Wan, C. L. Lin, X. B. Yu and T. H. Wang, Appl. Phys. Lett., 2004, 84, 124–126 CrossRef CAS PubMed; (c) A. Wei, X. W. Sun, C. X. Xu, Z. L. Dong, M. B. Yu and W. Huang, Appl. Phys. Lett., 2006, 88, 213102 CrossRef PubMed.
- (a) J. Cui, Y. Zheng, Z. Shen and X. Wan, Langmuir, 2010, 26, 15508–15515 CrossRef CAS PubMed; (b) N. Zweep, A. Hopkinson, A. Meetsma, W. R. Browne, B. L. Feringa and J. van Esch, Langmuir, 2009, 25, 8802–8809 CrossRef CAS.
- U. K. Das, D. R. Trivedi, N. N. Adarsh and P. Dastidar, J. Org. Chem., 2009, 74, 7111–7121 CrossRef CAS PubMed.
- E. Ostuni, P. Kamaras and R. G. Weiss, Angew. Chem., Int. Ed. Engl., 1996, 35, 1324–1326 CrossRef CAS.
- C. B. Aakeröy, B. M. T. Scott and J. Desper, New J. Chem., 2007, 31, 2044–2051 RSC.
- M. Iwaoka, S. Takemoto and S. Tomoda, J. Am. Chem. Soc., 2002, 124, 10613–10620 CrossRef CAS PubMed.
- A. Mantion, A. G. Guex, A. Foelske, L. Mirolo, K. M. Fromm, M. Painsid and A. Taubert, Soft Matter, 2008, 4, 606–617 RSC.
- L. Kumari and W. Z. Li, Cryst. Res. Technol., 2010, 45, 311–315 CrossRef CAS.
- (a) J.-L. Li, X.-Y. Liu, X.-G. Wang and R.-Y. Wang, Langmuir, 2011, 27, 7820–7827 CrossRef CAS PubMed; (b) S. Ray, A. K. Das and A. Banerjee, Chem. Commun., 2006, 2816–2818 RSC.
- M. George and R. G. Weiss, Chem. Mater., 2003, 15, 2879–2888 CrossRef CAS.
- Q. V. Dolomanov, L. B. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- G. Gundiah, S. Mukhopadhyay, U. G. Tumkurkar, A. Govindaraj, U. Maitra and C. N. R. Rao, J. Mater. Chem., 2003, 13, 2118–2122 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Gelation behaviour of compounds 1a–1h, 2a–2h and 3a–3h, TGA thermogram, photographic image of 1c–1h. CCDC 958233 (1c), 966725 (1d), 957407 (2d) and 965887 (3c). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4nj01247j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |