DNA-interacting and biological properties of copper(II) complexes from amidino-O-methylurea
Atittaya
Meenongwa
ab,
Rosa F.
Brissos
b,
Chaiyaporn
Soikum
c,
Prapansak
Chaveerach
c,
Patrick
Gamez
*bd,
Yanee
Trongpanich
e and
Unchulee
Chaveerach
*a
aMaterials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand. E-mail: sunchul@kku.ac.th; Fax: +66 43202373
bDepartament de Química Inorgànica, Universitat de Barcelona, Martí I Franqués 1-11, 08028 Barcelona, Spain. E-mail: patrick.gamez@qi.ub.es; Fax: +34 934907725
cDepartment of Veterinary Public Health, Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
dInstitució Catalana de Recerca i Estudis Avançats (ICREA), Passeig Lluís Companys, 23, 08010 Barcelona, Spain
eDepartment of Biochemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
First published on 14th November 2014
Abstract
The interaction of two copper(II) complexes, namely [Cu(L1)Cl2]2 (1) and [Cu(L2)Cl2]2 (2), where L1 = 1-amidino-O-methylurea and L2 = N-(benzyl)-amidino-O-methylurea, with DNA has been thoroughly investigated using different characterization techniques, including electronic absorption spectroscopy, viscosity measurements, fluorescence spectroscopy, circular dichroism spectroscopy, thermal denaturation, stoichiometric determination, gel electrophoresis and atomic-force microscopy. The coordination compounds exhibit DNA binding potential by non-intercalation and DNA-cleaving ability through the oxidative pathway. Indeed, both complexes display antibacterial properties (against three bacteria involved in human-food poisoning, i.e. Salmonella, E. coli and Campylobacter). Furthermore, their cytotoxicity has been tested against three cancer cell lines, which are the small cell lung carcinoma (NCI-H187), the oral cavity carcinoma (KB) and the breast adenocarcinoma (MCF-7) and it was revealed that they are more cytotoxic than cisplatin against the NCI-H187 cancer cell line.
1. Introduction
The application of metal coordination compounds in biology has received a great deal of attention from the scientific community, because the use of chemical approaches and small molecules to control or restore (altered) biological processes has shown great potential. For instance, inorganic small molecules that can bind to DNA via two possible binding modes, covalent bonds and non-covalent interactions (i.e. intercalation and non-intercalation) are of paramount importance to medicinal chemistry. DNA has become a privileged target for metal-based therapeutic agents,1 after the great impact of the square-planar anticancer agent [Pt(NH3)2Cl2], viz. cisplatin,2 in the treatment of various cancers, such as testicular, ovarian, head and neck as well as small cell lung cancers.3 The molecular mechanism of cisplatin is believed to involve in the coordination with the N7 guanine, leading to apoptosis.4 However, treatment with cisplatin causes severe side effects and (acquired) cell resistance to the drug representing a significant problem; therefore, the design and development of new metal complexes exhibiting higher efficiencies, lower toxicities and target-specific properties is required. Hence, numerous platinum and some non-platinum (ruthenium, gold, cobalt, iron, zirconium and copper) compounds have been described in the literature together with their cytotoxicity behavior.5–12 In particular, complexes based on copper, which is an important trace element in the human body involving essential enzymatic functions due to its Cu(II)/Cu(I) redox process,13 have attracted the attention of researchers worldwide.14–17 Moreover, copper is cheaper and gives the complexes with diverse geometries. Therefore, copper is suitable to be an alternative element providing the benefits in both design and various applications. A number of copper(II) coordination compounds have thus shown some interesting DNA-binding and cleavage properties, as well as antibacterial activities.12,18–22Over the past few years, we have been focusing on the development of copper(II) complexes from amidino-O-alkylurea ligands to increase their biological properties. This ligand system shows high potential to form hydrogen-bonding interactions,23 which are of paramount importance in the biological system and significantly useful in designs of DNA-interacting agents. There are several copper(II) coordination compounds prepared from amidino-O-alkylurea derivatives including 1-n-butylamidino-O-alkylurea, 1-phenylamidino-O-methylurea, 1-phenylamidino-O-i-butylurea and 1-amidino-O-2-alkoxyethylurea as described in the literature, which display interesting biological properties such as DNA-binding abilities, or activities against fungal and bacterial pathogens.24–26 These evidences inform the great potential of such compounds for medicinal applications. Herein, two copper(II) complexes of 1-amidino-O-methylurea (L1) and N-(benzyl)-amidino-O-methylurea (L2) have been prepared which formed the dimeric copper(II) complexes [Cu(L1)Cl2]2 (1) and [Cu(L2)Cl2]2 (2) (Fig. 1) and adopted an approximate square-pyramidal geometry.27 Their binding ability to nucleobases has been previously investigated and found that they are indeed capable of binding to the N3 cytosine by the replacement of the chloride ligands.28 In addition, the related monomeric copper(II) complexes [Cu(L1)2Cl2] and [Cu(L2)2Cl2] with a square-planar geometry29 have also been reported to exhibit potential DNA-interacting properties and antimicrobial activities against Campylobacter.30 To continue our research, the complexes 1 and 2 have been intensively studied in terms of the DNA interacting and biological properties. Furthermore, a difference in the N-substituted sidearm, –NH2 and –NHCH2Ph of the L1 and L2 ligands, respectively, has been examined the effect on both properties.
In the present study, the potential DNA interactions and antiproliferative behavior of the dimeric complexes 1 and 2 have been examined. Several techniques including electronic absorption titration, viscosity measurements, fluorescence spectroscopy, circular dichroism spectroscopy, thermal analysis and stoichiometric determination have been employed to monitor their prospective interaction with calf thymus DNA (CT-DNA). Their potential DNA-cleaving abilities have been investigated by gel electrophoresis and atomic-force microscopy (AFM), using pBR322 plasmid DNA. Their antimicrobial properties against three Gram-negative bacteria (namely Salmonella, E. coli and Campylobacter) have been determined applying the agar-well diffusion method. In addition, their cytotoxicity towards three human cancer cell lines, i.e. small cell lung carcinoma (NCI-H187), epidermoid carcinoma of the oral cavity (KB) and breast adenocarcinoma (MCF-7) has been evaluated using the resazurin microplate assay (REMA).
2. Experimental
2.1. Materials and instrumentation
The sodium salt of calf thymus DNA (CT-DNA, Type I fibrous) was purchased from Sigma-Aldrich. Plasmid pBR322 DNA (4361 bp, 0.25 μg μL−1) was obtained from Bio Basic INC and Roche Farma, S.A. Ethidium bromide (EB) solution (10 mg mL−1) and tris(hydroxymethyl)aminomethane (Tris base) were purchased from Promega. Agarose (D-1, Low EEO) was purchased from Pronadisa. N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) was obtained from Sigma-Aldrich. All reagents were of molecular biology grade and used without further purification. The coordination compounds [Cu(L1)Cl2]2 (1) and [Cu(L2)Cl2]2 (2) were prepared as reported earlier.28Electronic absorption spectra were recorded using an Agilent 8453 UV-Vis spectrophotometer. Fluorescence determination was performed on a Shimadzu RF–5301PC spectrofluorophotometer. Circular dichroism (CD) spectra were recorded on a Jasco J-810 spectropolarimeter. The amount of copper for each stoichiometric determination was determined using a Perkin Elmer AAnalyst 100 atomic absorption spectrometer. The electrophoretic band intensities were visualized with a Bio-Rad Gel Doc 2000 system using the LABWORK software. The AFM images were obtained by using a Nanoscope V Multimode 8 AFM (Bruker AXS) operating in the PEAK FORCE tapping mode. Commercial Si-tip on Nitride lever cantilevers (SNL, Bruker) with a force constant of 0.4 N m−1 was used.
2.2. DNA-binding studies
The DNA stock solution, prepared in Tris-buffer (containing 5 mM Tris-HCl and 50 mM NaCl at pH 7.1), gave a UV-absorbance ratio A260/A280 of about 1.8–1.9 (where A260 and A280 are the absorbances of a DNA sample at 260 and 280 nm, respectively), indicating that DNA was sufficiently free of protein contamination.31 The DNA stock solution was kept at 4 °C and used within 4 days. A 10-fold dilution of the DNA concentration was determined spectrophotometrically at 260 nm, by using the molar extinction coefficient value of 6600 M−1 cm−1.32 The stock solutions of 1 and 2 prepared in Tris-buffer were highly soluble and showed the similar blue color to their solid phase, thus, suggesting the stability of both compounds under these conditions. The stock solution was freshly prepared before utilization. All experiments were carried out in Tris-buffer at pH 7.1, except for the thermal denaturation study in HEPES-buffer (containing 40 mM HEPES and 10 mM MgCl2 at pH 7.1).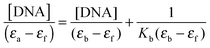 | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 30 min). The supernatant was separated by pouring it out slowly. Next, deionized water (25 mL) was added to dissolve the DNA–copper(II) precipitate, and the DNA concentration was calculated (from triplicate experiments) by the absorption intensity at 259 nm using ε = 6600 M−1 cm−1.32 The amount of copper(II) was determined by atomic absorption spectroscopy, hence providing the Cu (mmol)/DNA (mol base) ratio.
000 rpm, 30 min). The supernatant was separated by pouring it out slowly. Next, deionized water (25 mL) was added to dissolve the DNA–copper(II) precipitate, and the DNA concentration was calculated (from triplicate experiments) by the absorption intensity at 259 nm using ε = 6600 M−1 cm−1.32 The amount of copper(II) was determined by atomic absorption spectroscopy, hence providing the Cu (mmol)/DNA (mol base) ratio.
2.3. Nuclease activity
| %DNA cleavage activity = {[(volume of SC-DNA)control − (volume of SC-DNA)sample]/(volume of SC-DNA)control} × 100 | (3) |
In the presence of ascorbic acid (100 μM), the complexes (10–600 μM) were incubated for 1 h and electrophoresed in 0.8% agarose gel in a 1× TAE buffer at 50 V for 1.5 h.
2.4. In vitro antibacterial activity
The screening of the in vitro antibacterial activity of the copper(II) complexes (50 mg mL−1) against three Gram-negative bacteria (Salmonella, E. coli and Campylobacter) was performed by the agar-well diffusion method.39 Active culture of the bacteria was transferred into 0.75% (w/w) semisolid brucella agar (10 mL) at 50 °C. Subsequently, the incubated medium was swirled to distribute the cell culture of bacteria and held at room temperature for 30 min. A well of 6 mm diameter was made aseptically. The plates were placed at 37 °C for 48 h under the appropriate conditions to allow the cell culture growth. Each complex was dissolved in water to give the final concentration of 0.50% and transferred into a well aseptically. The inhibitory activity of the complex on bacteria was obtained from a clearing zone around the disc. The minimum inhibitory concentration (MIC) values were also determined by the two-fold serial dilution method in liquid media containing the tested complexes.40 The experiments were carried out in duplicate.2.5. Anticancer activity assay
Cancer-cell growth inhibition tests were carried out against three human cancer cell lines, namely the KB (epidermoid carcinoma of oral cavity, ATCC CCL-17), MCF-7 (breast adenocarcinoma, ATCC HTB-22) and NCI-H187 (small cell lung carcinoma, ATCC CRL-5804) cell lines, using the resazurin microplate assay (REMA), as described in the literature.41 Ellipticine, doxorubicin and tamoxifen were used as positive controls, and 0.5% DMSO was used as a negative control. To investigate the potential cytotoxic behaviors of the copper(II) complexes, cells at a logarithmic growth phase were harvested and diluted in fresh medium to 7 × 104 cells mL−1 for KB, and 9 × 104 cells mL−1 for MCF-7 and NCI-H187. Subsequently, 5 μL of the test sample diluted in 5% DMSO, and 45 μL of cell suspension were added to 384-well plates and then incubated at 37 °C in 5% CO2. After the incubation period (3 days for KB and MCF-7, and 5 days for NCI-H187), 12.5 μL of 62.5 μg mL−1 resazurin solution were added to each well, and the plates were then incubated at 37 °C for 4 h. The fluorescence signal was measured using a SpectraMax M5 multi-detection microplate reader (Molecular devices, USA) at the excitation and emission wavelengths of 530 nm and 590 nm, respectively. The inhibition percentage of the cell growth was calculated with eqn (4). | (4) |
3. Results and discussion
3.1. DNA-binding studies
DNA is the classical pharmacological target of metal-based anticancer agents; therefore, the study of the potential interaction of coordination compounds with DNA is of paramount importance for the development of molecules with potential medical applications. There are four possible ways in which small molecules can bind to double-stranded DNA; (i) intercalation between two adjacent base pairs and perpendicular to the helical axis; (ii) outside-edge binding to the sugar-phosphate backbone of the helix through electrostatic interactions; (iii) groove binding with functional groups into either the major or minor groove43 and (iv) the covalent interaction between DNA and metal complexes at the nitrogen atoms of nucleobases.4 Therefore, several characterization techniques have been employed to study the interactions of copper(II) complexes 1 and 2 with CT-DNA.The electronic spectra of 1 and 2 (Fig. 2) actually display intense absorption bands at λmax = 226 and 209 nm, respectively, which are assigned to π–π* intraligand transitions.30,46–48 Upon increasing the DNA concentration, both 1 and 2 show hyperchromism with a blue shift of 2 and 5 nm, respectively. Such a behavior suggests that both compounds most likely interact through a non-intercalative binding mode with DNA, probably via (i) hydrogen bonds between base pairs and the ligands; (ii) electrostatic attractions between the positively charged metal center and the negatively charged phosphate backbone and (iii) the coordination bonding of the copper(II) centers with cytosine nucleobases of DNA as previously reported.28 Furthermore, the comportments of 1 and 2 (Table 1) are comparable to those of related copper(II) compounds described in the literature.25,26,30
| Compound | λ max (nm) | Effect on intensity | Shift (nm) | K b (M−1) | Ref. |
|---|---|---|---|---|---|
| a L3 = 1-phenylamidino-O-methylurea. b L4 = 1-phenylamidino-O-i-butylurea, tmen = N,N,N′,N′-tetramethylethylenediamine. c L5 = 1-amidino-O-2-methoxyethylurea. d L6 = 1-amidino-O-2-ethoxyethylurea. e L7 = 1-amidino-O-2-buthoxyethylurea. | |||||
| Ethidium bromide (EB) | 285 | Hypochromism | +1 | 5.35 × 106 | This work |
| [Cu(L1)Cl2]2, (1) | 226 | Hyperchromism | −2 | 5.63 × 104 | This work |
| [Cu(L2)Cl2]2, (2) | 209 | Hyperchromism | −5 | 1.07 × 105 | This work |
| [Cu(L1)2]Cl2 | 228 | Hyperchromism | 0 | 5.67 × 104 | 30 |
| [Cu(L2)2]Cl2 | 229 | Hyperchromism | −1 | 1.16 × 105 | 30 |
| [Cu(L3)2(H2O)]2(Cl2)2a | 232 | Hyperchromism | 0 | 1.30 × 105 | 25 |
| [Cu(L4)(tmen)]2(Cl2)2·2H2Ob | 232 | Hyperchromism | 0 | 1.50 × 106 | 25 |
| [Cu(L5)2](ClO4)2·H2Oc | 224 | Hyperchromism | −2 | 4.08 × 104 | 26 |
| [Cu(L6)2](ClO4)2·2/3H2Od | 224 | Hyperchromism | −2 | 1.39 × 104 | 26 |
| [Cu(L7)2](ClO4)2·H2Oe | 225 | Hyperchromism | −3 | 3.06 × 104 | 26 |
The intrinsic binding constants (Kb) are 5.63 × 104 M−1 for 1 and 1.07 × 105 M−1 for 2, hence indicating that 2 interacts stronger than 1 with DNA. This difference is probably due to the presence of phenyl rings in the ligand L2, which may provide additional π-stacking interactions with DNA bases. The DNA-binding affinities of 1 and 2 are apparently lower than that of the intercalating agent EB (Table 1); however, it should be mentioned that the copper(II) compounds may interact with a different binding mode (not as an intercalator). Although, the electronic absorption studies have confirmed that the complexes 1 and 2 can bind to DNA, it is necessary to carry out further experiments to prove the binding mode.
| CuL + DNA–EB ⇌ DNA–CuL + EB K | (i) |
| CuL + DNA–EB ⇌ EB–DNA–CuL K′ | (ii) |
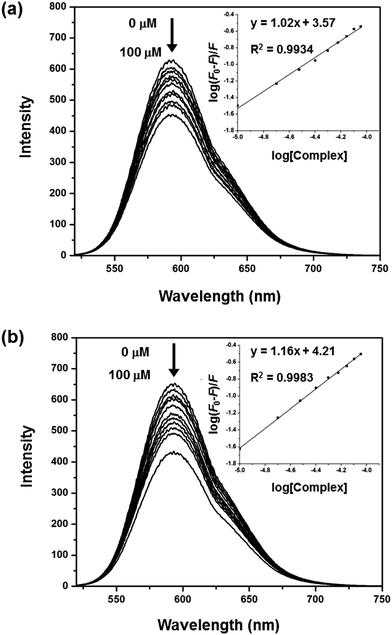
The UV-Vis studies and viscosity measurements suggest that both 1 and 2 most likely interact with DNA by non-intercalative modes. Hence, the main quenching process of the DNA–EB complex by the copper(II) compounds probably involves the formation of a non-fluorescent [DNA–EB–copper complex] species, i.e. pathway (ii), through a so-called fluorescence static quenching process. The association binding constant (Ka) and the number of equivalent binding sites (n) on the biomolecule can be calculated using eqn (2).35 The binding parameters for 1 and 2 are Ka = 3.73 × 103 M−1; n = 1.02 and Ka = 1.64 × 104 M−1; n = 1.16, respectively. These values indicate that the two complexes have distinct binding affinities towards DNA, as also suggested by the UV-Vis data (see Kb values above). Accordingly, 2 exhibits greater DNA-binding potential than 1.
 | ||
| Fig. 6 DNA melting curves of CT-DNA (100 μM) (■) in the absence and presence of 1 (a) and 2 (b), at the [Complex]/[DNA] ratios of 0.5 (●), 1.0 (▲), 1.5 (▼) and 2.0 (♦). | ||
The Tm value of free CT-DNA is 82.1 °C. Upon increase of the complex concentration, a slight increase of Tm is observed. The corresponding ΔTm values are in the range of 0.5–1.2 °C for 1, and 0.3–1.3 °C for 2. The low ΔTm values (close to zero) suggest that the DNA-binding mode of the complexes is non-intercalative.
3.2. Nuclease activity of the complexes towards plasmid pBR322 DNA
The potential interacting/cleaving properties of 1 and 2 were assessed by electrophoretic mobility measurements with plasmid pBR322 DNA. Fig. 7 shows the results of electrophoretic separations of the plasmid DNA induced by the different concentrations of the complexes 1 (Fig. 7a) and 2 (Fig. 7b) after 24 h for incubation and their behaviors are also comparable. Band intensities of Form II are significantly enhanced when treated by 1 and 2 from 200 μM to 400 μM (lanes 2–3), indicating that both complexes can cleave the supercoiled form (Form I) to nicked form (Form II). In the concentration from 600 μM to 1000 μM (lanes 4–6), both Forms I and II gradually decrease, suggesting the degradation of plasmid DNA into undetectable single-stranded pieces. Their cleaving behaviors at a large amount differ from the previous reports in which square planar copper(II) complexes ([Cu(L1)2]Cl2 and [Cu(L2)2]Cl2) show DNA cleavage from Form I to Form II.30 Band intensities were measured to determine the amount of each DNA form. The percentage of DNA cleavage activity (Fig. 8) was calculated by eqn (3). It is found that complex 2 shows considerably higher DNA cleavage efficiency than 1, demonstrating that 2 displays better cleaving activity than 1.
It is known that the DNA cleaving potential of copper(II) complexes can be enhanced by adding the exogenous agents such as hydrogen peroxide and ascorbic acid (H2ASC), which act as reducing agents. Herein, H2ASC is used to mimic the reducing environment that can be found inside the cells. Hence, H2ASC will induce the formation of copper(I) species (from 1 and 2), which would allow the potential formation of reactive oxygen species (ROS) that are able to cleave DNA. When H2ASC is added, completely different circumstances are achieved (Fig. 9). The corresponding H2ASC (100 μM) does not affect free DNA (lane 2). In the presence of H2ASC, Form II can be observed at the low concentration (10 μM) for both complexes. Moreover, Form III is also found at 50 μM for 1 (lane 7, Fig. 9a) and 40–50 μM for 2 (lanes 6, 7, Fig. 9b). At 60–100 μM (lanes 8–12), both 1 and 2 are capable of inducing the DNA degradation of plasmid DNA into very small single-stranded pieces which are observed as smear on gel. Upon enhancing the concentration of both from 200 μM to 600 μM (lanes 13–15), very strange features can be observed; indeed, the bands ascribed to Forms II and III reappear and increase.
As a result, it is preliminarily suggested that both 1 and 2 at concentrations ≤100 μM with H2ASC (100 μM) are capable of promoting DNA cleavage through an oxidative DNA damage pathway which gives the reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical (OH˙), singlet oxygen (1O2) or superoxide (O2−) that cleave DNA.58 The roles of copper(II) complexes showing their enzymatic behaviors can be explained by the proposed mechanism of in situ generation of H2O2 by oxidation of the cuprous complex under aerobic conditions in the presence of ascorbic acid acting as a reducing agent as shown below.59
| H2ASC + H2O ⇌ HASC− + H3O+ | (iii) |
| HASC− + 2Cu(II) → ASC + 2Cu(I) + H+ | (iv) |
| 2Cu(I) + O2 + 2H+ → 2Cu(II) + H2O2 | (v) |
| HASC− + O2 + H+ → ASC + H2O2 | (iv) + (v) |
The first step (iii) is the hydrolysis of ascorbic acid (H2ASC) generating the ascorbate form (HASC−), the second step (iv) is the reduction of the Cu(II) complex to the Cu(I) complex by HASC−. Then, the reaction of the cuprous state with dioxygen (v) leads to the generation of H2O2. Overall reactions (iv) + (v) correspond to the autooxidation of ascorbate which may involve a single two-electron oxidation step.60 Hydrogen peroxide then reacts with another equivalent of the Cu(I) through the Fenton reaction (vi) which gives the reactive species, hydroxyl radicals, responsible for DNA strand scission.59
| Cu(I) + H2O2 → Cu(II) + OH− + OH˙ | (vi) |
Hence, an excess amount of the copper(II) complexes (200–600 μM) cannot be reduced by the limited amount of ascorbic acid and may induce several undesirable secondary reactions. One of them is the formation of Cu(OH)2, which may precipitate, resulting in the reduction of DNA cleavage efficiency. The unusual phenomenon that is the reverse DNA cleavage altering from the Form III to Form II most likely arised from the fact that the concentration of copper(II) complexes (200–600 μM) is huge when compared with H2ASC (100 μM). This situation has also been found in the Fenton reaction catalyzed by ferrous ions which produced Fe(OH)3 precipitates due to ferrous ion excess.61,62 In summary, complexes 1 and 2 are both capable of cleaving plasmid DNA through the oxidative pathway and 2 also shows better activity than 1 in both with and without reducing agents corresponding to their DNA binding capabilities. The reason for this may arise from the presence of phenyl rings on ligand L2 which possibly gives additional binding positions on DNA.
3.3. In vitro antibacterial activity
According to the evidence showing DNA binding and cleaving behaviors of the copper(II) complexes, it guided us that both 1 and 2 may inhibit the growth of bacteria. Some copper(II) complexes containing amidino-O-alkylurea derivatives show antibacterial activities towards Bacillus,24E. coli, K. pneumonia and P. mirabilis,25 and Campylobacter.30 Therefore, the potential antibacterial activities of complexes 1 and 2 against Salmonella, E. coli and Campylobacter (which are known as human-food poisoning bacteria) have been examined. The corresponding results are presented as inhibition zone diameters (IZDs) and minimum inhibitory concentrations (MICs) (Table 3). Complexes 1 and 2 are capable of inhibiting the growth of all tested bacteria. Hence, 1 exhibits MIC values of 25.00 mg mL−1 for Salmonella and E. coli. Its inhibition activity is significantly higher towards Campylobacter, with a MIC value of 3.12 mg mL−1. These data perfectly correlate with the corresponding IZDs; indeed, the larger inhibition zone is achieved with Campylobacter, which indicates that 1 is more active against this bacterium. For complex 2, the MIC values obtained suggest that its antibacterial properties follow the order Campylobacter > Salmonella > E. coli. Interestingly, the antibacterial activity of 2 towards Salmonella is greater than that of 1, which is in agreement with the better DNA-binding and cleavage properties of 2. Finally, it should be mentioned that dimeric [Cu(L1)Cl2]2 (1) and [Cu(L2)Cl2]2 (2) are less efficient against Campylobacter than the corresponding monomeric complexes [Cu(L1)2Cl2] and [Cu(L2)2Cl2] which have been previously reported,30 it is probably because the latter display slightly better DNA-interacting properties. Enrofloxacin antibacterial drug was also tested as a reference. However, the results indicate that both 1 and 2 may be applied for the growth inhibition of a series of bacteria involved in human-food poisoning. Although, their obtained antibacterial activities expressed by MIC values are in the mg mL−1 (mM) range and much too large to practical uses when compared with enrofloxacin, it is the preliminary data leading us to the development of the metal complexes to be the antibacterial agents.| Compound | Inhibition-zone diameter (mm) | MIC (mg mL−1) | ||||
|---|---|---|---|---|---|---|
| Salmonella | E. coli | Campylobacter | Salmonella | E.coli | Campylobacter | |
| a Data from ref. 30. b N.D. = not determined. | ||||||
| [Cu(L1)Cl2]2, (1) | 11.0 | 12.0 | 22.5 | 25.00 | 25.00 | 3.12 |
| [Cu(L2)Cl2]2, (2) | 15.0 | 11.0 | 18.0 | 12.50 | 25.00 | 3.12 |
| [Cu(L1)2]Cl2a | N.D.b | N.D.b | 9.0 | — | — | 1.52 |
| [Cu(L2)2]Cl2a | N.D.b | N.D.b | 14.5 | — | — | 0.78 |
| Enrofloxacin | 10.0 | 10.0 | 15.0 | 0.002 | 0.008 | 0.015 |
3.4. Cytotoxicity assays
The positive results obtained from the DNA-interactions of the copper(II) complexes encouraged us to investigate their in vitro cytotoxicity against three human cancer cell lines, namely the small cell lung carcinoma (NCI-H187), the oral cavity carcinoma (KB) and the breast adenocarcinoma (MCF-7) by resazurin microplate assay. The corresponding IC50 values are summarized in Table 4.Both 1 and 2 can inhibit the growth of NCI-H187 cells with similar IC50 values (Table 4), but are inactive towards MCF-7 cells. For the KB cell line, only complex 2 shows some activity, with an IC50 value of 22.51 μg mL−1. The better cytotoxicity of complex 2 corroborates the stronger DNA binding ability compared to complex 1. The existence of phenyl rings on the ligand L2 may serve additional interactions providing better reactivity for the complex 2. Moreover, their anticancer activities have also been compared with cisplatin and the related monomeric compounds [Cu(L1)2]Cl2 and [Cu(L2)2]Cl2. Under this condition, cisplatin is capable of inhibiting only the growth of KB cells with less cytotoxicity than 2. On the other hand, though the compounds [Cu(L1)2]Cl2 and [Cu(L2)2]Cl2 appear to have slightly higher DNA binding potential than 1 and 2, respectively, (see Table 1), they are not active against all tested cell lines. Reasons for the different anticancer behaviors of the monomeric and dimeric copper(II) compounds can be explained by the structures and compared with cisplatin. For the monomeric compounds, after losing chloride anions by hydrolysis, the hydrolyzed forms, [Cu(L1)2]2+ and [Cu(L2)2]2+, which show the square-planar structures like cisplatin,29 are inactive, probably due to the CuN4 chromophore in which the copper(II) center is fixed by coordination with the two N,N-bidentate ligands, L1 and L2, thus it cannot further bind with DNA nucleobases via coordinative bonds as cisplatin. In the case of 1 and 2 which are dimeric compounds containing square-pyramidal geometry around the copper(II) center (Fig. 1), there are two terminal chloride ligands available to be replaced and then interact with DNA like cisplatin.4 These assays thus indicate that the copper(II) compounds of the type 1 and 2 may find applications as potential cytotoxic agents for specific cancer cell lines (such as the KB cell line).
4. Conclusions
Two copper(II) complexes [Cu(L1)Cl2]2 (1) and [Cu(L2)Cl2]2 (2) from hydrophilic ligands, namely amidino-O-methylurea derivatives, have been prepared and their DNA-interacting properties have been assessed. Both complexes have DNA binding potential through non-intercalation such as hydrogen bonds, electrostatic interactions and partial intercalation. In addition, these water-soluble complexes are efficient DNA cleavers and act as antibacterial agents, being lethal for a series of prokaryotic microorganisms involved in human-food poisoning, viz. Salmonella, E. coli and Campylobacter. Moreover, these metal-based molecules exhibit cytotoxic properties towards NCI-H187 cancer cells (small cell lung carcinoma), which are not affected by the well-known cisplatin. Complex 2 containing aromatic rings on sidechains of the ligands L2 shows the greater DNA binding and cleaving efficiencies, thus revealing higher antibacterial activity against Campylobacter as well as they are more effective than complex 1 to inhibit the growth of cancer cell types such as the small cell lung carcinoma (NCI-H187) and the oral cavity carcinoma (KB). Such a result has pointed out that the functional groups on the sidechain of the ligands have a significant effect on the DNA-interacting and biological properties of metal complexes.The present study therefore shows high potential of (amidino-O-methylurea)-based complexes for biological applications. Indeed, functional groups can be easily introduced into amidino-O-methylurea derivatives (see R group in Fig. 1), thus providing an easy access to numerous water-soluble ligands (which is of paramount importance for biological applications) that can bind biometals like copper and generate potentially bioactive metal agents.
Acknowledgements
This work was financially supported by Center of Excellence for Innovation in Chemistry (PERCH-CIC) and the Development and Promotion of Science and Technology Talents Projects (DPST) (to A.M.). We also thank the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Communication, through the Advanced Functional Materials Cluster of Khon Kaen University. P.G. acknowledges ICREA (Institució Catalana de Recerca i Estudis Avançats), the Ministerio de Economía y Competitividad of Spain (Project CTQ2011-27929-C02-01), and the support of COST Actions CM1003 and CM1105.References
- Metal-DNA Chemistry, ACS Symposium Series, ed. T. D. Tullius, American Chemical Society 402, Washington DC, 1989, pp.1–23 Search PubMed.
- B. Rosenberg, L. van Camp, J. E. Trosko and V. H. Mansour, Nature, 1969, 222, 385–386 CrossRef CAS.
- P. J. Loehrer and L. H. Einhorn, Ann. Intern. Med., 1984, 100, 704–713 CrossRef CAS PubMed.
- A. Eastman, in Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug, ed. B. Lippert, VHCA & Wiley-VCH, Zurich & Germany, 1999, pp. 111–134 Search PubMed.
- Y.-Y. Xie, H.-L. Huang, J.-H. Yao, G.-J. Lin, G.-B. Jiang and Y.-J. Liu, Eur. J. Med. Chem., 2013, 63, 603–610 CrossRef CAS PubMed.
- S. David, R. S. Perkins, F. R. Fronczek, S. Kasiri, S. S. Mandal and R. S. Srivastava, J. Inorg. Biochem., 2012, 111, 33–39 CrossRef CAS PubMed.
- M. N. Patel, B. S. Bhatt and P. A. Dosi, Inorg. Chem. Commun., 2013, 29, 190–193 CrossRef CAS PubMed.
- S. D. Schimler, D. J. Hall and S. L. Debbert, J. Inorg. Biochem., 2013, 119, 28–37 CrossRef CAS PubMed.
- D. Wallis, J. Claffey, B. Gleeson, M. Hogan, H. Müller-Bunz and M. Tacke, J. Organomet. Chem., 2009, 694, 828–833 CrossRef CAS PubMed.
- A. Esparza-Ruiz, C. Herrmann, J. Chen, B. O. Patrick, E. Polishchuk and C. Orvig, Inorg. Chim. Acta, 2012, 393, 276–283 CrossRef CAS PubMed.
- F. A. Beckford, J. Thessing, A. Stott, A. A. Holder, O. G. Poluektov, L. Li and N. P. Seeram, Inorg. Chem. Commun., 2012, 15, 225–229 CrossRef CAS PubMed.
- I. Ali, W. A. Wani, K. Saleem and M.-F. Hseih, Polyhedron, 2013, 56, 134–143 CrossRef CAS PubMed.
- B. R. Stern, J. Toxicol. Environ. Health, Part A, 2010, 73, 114–127 CrossRef CAS PubMed.
- S. J. Lippard and J. M. Berg, Principles of bioinorganic chemistry, University Science Books, Mill Valley, California, 1994 Search PubMed.
- M. Jagadeesh, S. K. Kalangi, L. S. Krishna and A. V. Reddy, Spectrochim. Acta, Part A, 2014, 118, 552–556 CrossRef CAS PubMed.
- S. Sayen, A. Carlier, M. Tarpin and E. Guillon, J. Inorg. Biochem., 2013, 120, 39–43 CrossRef CAS PubMed.
- B. Duff, V. R. Thangella, B. S. Creaven, M. Walsh and D. A. Egan, Eur. J. Pharmacol., 2012, 689, 45–55 CrossRef CAS PubMed.
- G.-Y. Li, K.-J. Du, J.-Q. Wang, J.-W. Liang, J.-F. Kou, X.-J. Hou, L.-N. Ji and H. Chao, J. Inorg. Biochem., 2013, 119, 43–53 CrossRef CAS PubMed.
- V. C. Silveira, H. Benezra, J. S. Luz, R. C. Georg, C. C. Oliveira and A. M. C. Ferreira, J. Inorg. Biochem., 2011, 105, 1692–1703 CrossRef PubMed.
- M. N. Patel, P. A. Dosi, B. S. Bhatt and V. R. Thakkar, Spectrochim. Acta, Part A, 2011, 78, 763–770 CrossRef PubMed.
- A. Kellett, O. Howe, M. O′Connor, M. McCann, B. S. Creaven, S. McClean, A. F.-A. Kia, A. Casey and M. Devereux, Free Radical Biol. Med., 2012, 53, 564–576 CrossRef CAS PubMed.
- S. Kashanian, M. M. Khodaei, H. Roshanfekr, N. Shahabadi and G. Mansouri, Spectrochim. Acta, Part A, 2012, 86, 351–359 CrossRef CAS PubMed.
- P. Hubberstey, U. Suksangpanya and C. Wilson, CrystEngComm, 2000, 26, 141–145 RSC.
- O. I. Singh, M. Damayanti, N. R. Singh, R. K. H. Singh, M. Mohapatra and R. M. Kadam, Polyhedron, 2005, 24, 909–916 CrossRef CAS PubMed.
- S. P. Devi, R. K. B. Devi, M. Damayanti, N. R. Singh, R. K. H. Singh and R. M. Kadam, J. Coord. Chem., 2011, 64, 1586–1601 CrossRef CAS.
- S. P. Devi, R. K. B. Devi, N. S. Devi, L. J. Singh and R. K. H. Singh, Polyhedron, 2012, 47, 1–8 CrossRef PubMed.
- M. J. Begley, P. Hubberstey and C. H. M. Moore, J. Chem. Res., Synop., 1986, 172–173 CAS.
- A. Meenongwa, U. Chaveerach and K. Siriwong, Inorg. Chim. Acta, 2011, 366, 357–365 CrossRef CAS PubMed.
- U. Suksangpanya, A. J. Blake, P. Hubberstey, D. J. Parker, S. J. Teat and C. L. Wilson, CrystEngComm, 2003, 5, 10–22 RSC.
- U. Chaveerach, A. Meenongwa, Y. Trongpanich, C. Soikum and P. Chaveerach, Polyhedron, 2010, 29, 731–738 CrossRef CAS PubMed.
- J. Marmur, J. Mol. Biol., 1961, 3, 208–218 CrossRef CAS.
- M. F. Reichmann, S. A. Rice, C. A. Thomas and P. Doty, J. Am. Chem. Soc., 1954, 76, 3047–3053 CrossRef CAS.
- A. Wolfe, G. H. J. Shimer and T. Meehan, Biochemistry, 1987, 26, 6392–6396 CrossRef CAS.
- G. Cohen and H. Eisenberg, Biopolymers, 1969, 8, 45–55 CrossRef CAS.
- X.-Z. Feng, Z. Lin, L.-J. Yang, C. Wang and C. C.-L. Bai, Talanta, 1998, 47, 1223–1229 CrossRef CAS.
- G. Facchin, E. Kremer, D. A. Barrio, S. B. Etcheverry, A. J. Costa-Filho and M. H. Torre, Polyhedron, 2009, 28, 2329–2334 CrossRef CAS PubMed.
- M. F. Shubsda, J. Goodisman and J. C. Dabrowiak, J. Biochem. Biophys. Methods, 1997, 34, 73–79 CrossRef CAS.
- M. N. Patel, P. A. Dosi and B. S. Bhatt, Polyhedron, 2010, 29, 3238–3245 CrossRef CAS PubMed.
- C. H. Collins and P. M. Lyne, Microbiological Methods, University Park Press, Baltimore, 1970, p. 422 Search PubMed.
- E. J. L. Lana, F. Carazza and J. A. Takahashi, J. Agric. Food Chem., 2006, 54, 2053–2056 CrossRef CAS PubMed.
- J. O′Brien, I. Wilson, T. Orton and F. Pognan, Eur. J. Biochem., 2000, 267, 5421–5426 CrossRef.
- M. J. O'Neill, D. H. Bray, P. Boardman, J. D. Phillipson and D. C. Warhurst, Planta Med., 1985, 51, 394–398 CrossRef PubMed.
- G. M. Blackburn, M. J. Gait and D. Loakes, in Nucleic Acid in Chemistry and Biology, ed. D. M. Williams, RSC Publishing, Cambridge, 3rd edn, 2006, pp. 342–382 Search PubMed.
- J. M. Kelly, A. B. Tossi, D. J. McConnell and C. OhUigin, Nucleic Acids Res., 1985, 13, 6017–6034 CrossRef CAS PubMed.
- Q. Li, P. Yang, H. Wang and M. Guo, J. Inorg. Biochem., 1996, 64, 181–195 CrossRef CAS.
- Y.-J. Liu, X. Y. Wei, F.-H. Wu, W.-J. Mei and L.-X. He, Spectrochim. Acta, Part A, 2008, 70, 171–176 CrossRef PubMed.
- S. Tabassum, R. A. Khan, F. Arjmand, M. Aziz, A. S. Juvekar and S. M. Zingde, Carbohydr. Res., 2011, 346, 2886–2895 CrossRef CAS PubMed.
- F. Arjmand, S. Parveen, M. Afzal and M. Shahid, J. Photochem. Photobiol., B, 2012, 114, 15–26 CrossRef CAS PubMed.
- S. Satyanarayana, J. C. Dabrowiak and J. B. Chaires, Biochemistry, 1992, 31, 9319–9324 CrossRef CAS.
- B. C. Baguley and M. LeBret, Biochemistry, 1984, 23, 937–943 CrossRef CAS.
- R. F. Pasternack, M. Cacca, B. Keogh, T. A. Stephenson, A. P. Williams and F. J. Gibbs, J. Am. Chem. Soc., 1991, 113, 6835–6840 CrossRef CAS.
- E. Nyarko, N. Hanada, A. Habib and M. Tabata, Inorg. Chim. Acta, 2004, 357, 739–745 CrossRef CAS PubMed.
- V. I. Ivanov, L. E. Minchenkova, A. K. Schyolkina and A. I. Poletayev, Biopolymers, 1973, 12, 89–110 CrossRef CAS PubMed.
- B. Norden and F. Tjerneld, Biopolymers, 1982, 21, 1713–1734 CrossRef CAS PubMed.
- E. K. Efthimiadou, Y. Sanakis, M. Katsarou, C. P. Raptopoulou, A. Karaliota, N. Katsarosa and G. Psomas, J. Inorg. Biochem., 2006, 100, 1378–1388 CrossRef CAS PubMed.
- G. A. Neyhart, N. Grover, S. R. Smith, W. Kalsbeck, T. A. Fairley, M. Cory and H. H. Thorp, J. Am. Chem. Soc., 1993, 115, 4423–4428 CrossRef CAS.
- G. L. Eichhorn and Y. A. Shin, J. Am. Chem. Soc., 1968, 90, 7323–7328 CrossRef CAS.
- B. Halliwell and J. M. C. Gutteridge, Free Radicals Biol. Med., Oxford Science Publication, Oxford, 3rd edn, 1999, pp. 42–43 Search PubMed.
- D. S. Sigman and C.-H. B. Chen, Acc. Chem. Res., 1986, 19, 180–186 CrossRef CAS.
- I. Yamazaki and L. H. Piette, Biochim. Biophys. Acta, 1961, 50, 62–69 CrossRef CAS.
- O. G. Rojas, C. G. Quintero, M. R. Bolívar, A. Romero and A. Rodríguez, CIMMACS '10 Proceedings of the 9th WSEAS international conference on computational intelligence, man-machine systems and cybernetics, Wisconssin, 2010, 251–258.
- J. Blanco, PhD thesis, Universidad Politécnica de Cataluña, Barcelona, España, 2009.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |