DOI:
10.1039/C4NJ01357C
(Paper)
New J. Chem., 2015,
39, 342-348
Three N-stabilized rhodamine-based fluorescent probes for Al3+via Al3+-promoted hydrolysis of Schiff bases†
Received
(in Montpellier, France)
12th August 2014
, Accepted 15th October 2014
First published on 15th October 2014
Abstract
Three probes for Al3+ detection by both fluorescence and the naked eye in acetonitrile solution were developed. The mechanism of fluorescence was based on the aluminum complexation with rhodamine and subsequent Al3+-promoted hydrolysis of the Schiff base. Theoretical calculations indicated that the aluminum complexes tend to hydrolyze to compound L6. A simple paper test strip system for the rapid monitoring of Al3+ was developed, indicating its convenient use in environmental samples.
Introduction
As is known to all, aluminum is the third most abundant element in the earth's crust. It widely exists in the environment due to acidic rain and is considered to be toxic in biological activities.1–3 Regarding toxicological effects of aluminum, its primary targets are different from those of heavy metals.4 The widespread use of aluminum in water treatment, as a food additive, and in many industrial activities including the manufacturing of cars and computers often exposes people to this metal.5,6 Excessive exposure of the human body to Al3+ leads to the malfunction of the central nervous system such as Alzheimer's disease and Parkinson's disease.7,8 The WHO has recommended the average daily human intake of Al3+ to be around 3–10 mg and the weekly tolerable dietary intake as 7 mg kg−1 body weight. Therefore, trace level determination of Al3+ is highly important.
In the last decade, many methods such as graphite furnace atomic absorption spectrometry, atomic emission spectrometry, inductively coupled plasma atomic emission spectrometry and electrochemical methods have been used for aluminum detection. All of these techniques are generally time-consuming and expensive as well. In contrast, optical detection via fluorescence is an operationally easy technique besides being highly sensitive. Due to great changes in the absorption and/or fluorescence spectra of many organic molecules after coordinating with ions, colorimetric and fluorescence analytical methods are very good and effective ways to detect metal ions.9–15
The rhodamine moiety has been widely used in the field of chemosensors, especially as a chemodosimeter, given its fluorescence off–on behavior that results from its particular structural properties. Upon metal binding, its structure can undergo a change from the spirolactam to an open ring amide, resulting in a magenta-colored, highly fluorescent compound.16
While some rhodamine-based chemosensors for Al3+ ions have been reported to date,17–26 dual colorimetric and fluorescent chemosensors for Al3+ are still rare and some of them are not efficient enough to be selective toward Al3+ or sensed in organic solvents. Therefore, developing sensors which are able to detect Al3+ by both fluorescence and the naked eye in aqueous solution are very valuable. In this paper, we report three new N-stabilized rhodamine-based fluorescent probes which exhibited sensitive detection toward Al3+via significant fluorescence enhancement in solution, and, at the same time, showed a significant color change from being colorless to red. Similar structures had been designed for compounds L1, L2 and L3. By comparing with each other, we could know that the different electronic distribution among the chemodosimeters' structures had great influence in their recognition toward Al3+. Owing to a more stable structure, L1 showed the best sensing property, so we selected L1 as a representative example to expatiate in the following discussion. To the best of our knowledge, none of the rhodamine-based probes whose fluorescent mechanism is based on the Al3+-promoted hydrolysis of the Schiff base has been reported for detection of Al3+ up to now.
Experimental
Apparatus
Fluorescence spectra measurements were performed on a HITACHI F-4500 fluorescence spectrophotometer, and the excitation and emission wavelength band passes were both set to 5.0 nm. Absorption spectra were recorded on a UV-2102 double-beam UV/Vis spectrometer, Perkin Elmer precisely. NMR spectra were recorded on a Bruker DTX-400 spectrometer in CDCl3, using TMS as an internal standard. Mass spectral determination was carried on a HPLC Q-T of HR-MS.
Materials
All the materials for synthesis were purchased from commercial suppliers and used without further purification. The solutions of metal ions were prepared from their nitrate salts, except for FeCl3, FeCl2, CrCl3 and MnCl2. The metal ions were prepared as 10.00 mM in water solution.
Syntheses
As shown in Scheme 1, the compounds L1–3 were prepared by reacting L4 with an aromatic aldehyde. L4 was synthesized according to the literature.27
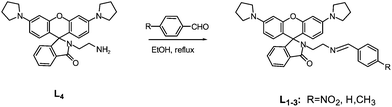 |
| | Scheme 1 Synthetic route of target compounds. | |
L1
.
L4 (0.5 mmol, 0.24 g) was dissolved in 25 mL ethanol, and then p-nitrobenzaldehyde (1 mmol, 0.15 g) was slowly added. The mixture was stirred and refluxed for 12 h at 80 °C. After distillation in vacuum, the residue was recrystallized with methanol and water to give the final product L1 in a yield of 78.4%. 1H NMR (400 MHz, CDCl3, δ ppm): 8.17 (d, J = 8.0 Hz, 2H), 8.06 (s, 1H), 7.91–7.90 (m, 1H), 7.72 (d, J = 8.4 Hz, 2H), 7.43 (s, 1H), 7.03 (d, J = 4.0 Hz, 1H), 6.44 (d, J = 8.4 Hz, 1H), 6.28 (s, 2H), 6.14–6,12 (m, 1H), 3.52–3.49 (m, 1H), 3.25 (s, 8H), 1.99 (s, 8H); 13C NMR (100 MHz, CDCl3, δ ppm): 168.5, 159.8, 153.9, 153.1, 148.8, 148.7, 141.7, 132.6, 130.9, 128.9, 128.8, 128.1, 123.7, 123.6, 122.8, 108.4, 105.7, 98.0, 65.2, 59.3, 47.6, 41.1, 25.5. HR-MS: C37H35N5O4 [M + H]+, calcd for 614.2762. Found: 614.2767 (Fig. S1–S3, ESI†).
L2
.
Compound L2 was prepared using a general procedure which is essentially similar to that used for L1. Yield of L2: 69.3%. 1H NMR (400 MHz, CDCl3, δ ppm): 8.01 (s, 1H), 7.92–7.90 (m, 1H), 7.58–7.56 (m, 2H), 7.44–7.39 (m, 2H), 7.36–7.31 (m, 3H), 7.05–7.03 (m, 1H), 6.46 (d, J = 8.8 Hz, 2H), 6.29 (s, 2H), 6.16–6.14 (m, 2H), 3.44 (s, 4H), 3.25 (t, J = 3.2 Hz, 8H), 2.01–1.98 (m, 8H); 13C NMR (100 MHz, CDCl3, δ ppm): 168.4, 162.4, 154.0, 153.1, 148.7, 132.4, 130.4, 128.9, 128.4, 128.1, 128.0, 123.7, 122.8, 108.4, 105.8, 98.0, 65.1, 59.0, 47.6, 41.3, 25.5. HR-MS: C37H36N4O2 [M + H]+, calcd for 569.2911. Found: 569.2910 (Fig. S4–S6, ESI†).
L3
.
The following compound was prepared using a general procedure which is essentially similar to that used for L1. Yield of L3: 62.7%. 1H NMR (400 MHz, CDCl3, δ ppm): 8.03 (s, 1H), 7.94–7.93 (m, 1H), 7.49 (d, J = 7.6 Hz, 2H), 7.45–7.43 (m, 2H), 7.15 (d, J = 7.6 Hz, 2H), 7.07–7.05 (m, 1H), 6.49 (d, J = 8.8 Hz, 2H), 6.32 (s, 2H), 6.17 (d, J = 8.4 Hz, 2H), 3.77–3.71 (m, 4H), 3.46–3.29 (m, 8H), 2.36 (s, 3H), 2.02 (s, 8H); 13C NMR (100 MHz, CDCl3, δ ppm): 168.3, 162.3, 154.1, 153.1, 148.7, 140.6, 133.7, 132.4, 131.1, 129.1, 128.9, 128.1, 127.9, 123.7, 122.8, 108.4, 105.8, 98.0, 65.1, 59.0, 47.6, 41.3, 25.5, 21.5. HR-MS: C38H38N4O2 [M + H]+, calcd for 583.3068. Found: 583.3074 (Fig. S7–S9, ESI†).
Results and discussion
The structure of title compounds L1, L2 and L3 were characterized by 1H NMR, 13C NMR and HR-MS spectrometry. The results were in good agreement with the structure shown in Scheme 1. Fluorescence and UV-vis studies were performed using a 10 μM solution of L1, L2 and L3 in a CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution with appropriate amounts of metal ions. Solutions were shaken for 120 min before measuring the absorption and fluorescence in order to make the metal ions chelate with the sensors sufficiently.
5, v/v) solution with appropriate amounts of metal ions. Solutions were shaken for 120 min before measuring the absorption and fluorescence in order to make the metal ions chelate with the sensors sufficiently.
Steady-state optical properties
Compared with L2 + Al3+ and L3 + Al3+, L1 + Al3+ had higher fluorescence quantum yield (Fig. S10, ESI†). This means that the strong electron withdrawing group on benzene is beneficial to the chelation of Al3+ with sensors. The effect of the reaction media on the binding of L1 with Al3+ was studied, and the results are shown in Fig. S11 (ESI†). It was found that the solvent has a great effect on the coordination reaction. When the coordination reaction was performed in acetonitrile–water solution, high F/F0 and ΔA values were obtained, indicating that acetonitrile–water media is favorable for fluorescence measurement. Therefore, acetonitrile–water solution was selected for both fluorescence assay and colorimetric assay. As shown in Fig. 1, L1 exhibited a 190-fold enhancement of fluorescence intensity at a peak wavelength λmax = 582 nm in the presence of 10 equiv. Al3+, so we selected L1 as the representative when expatiating on the characters of the three compounds in the following discussion.
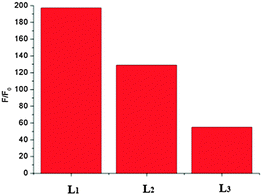 |
| | Fig. 1
F: fluorescence intensity (at 582 nm) of L1, L2 and L3 (10 μM) in CH3CN/H2O (95/5, v/v) in the presence of Al3+ (100 μM) (λex = 520 nm); F0: fluorescence intensity (at 582 nm) of L1, L2 and L3 (10 μM) only in CH3CN/H2O (95/5, v/v) (λex = 520 nm). | |
UV-vis spectral responses of L1
As shown in Fig. 2, the UV-vis spectrum of compound L1 (10 μM) exhibited only very weak bands over 400 nm. Addition of 10 equiv. Al3+ into solution immediately resulted in a significant enhancement of absorbance at about 562 nm simultaneously changing the color to red. Under identical conditions, no obvious response could be observed upon the addition of other ions including Zn2+, Mg2+, Ca2+, Cd2+, Cu2+, Pb2+, Hg2+, Ba2+, Ni2+, Fe2+, Mn2+, K+, Li+, Ag+, Co2+ and Na+ except for Fe3+ and Cr3+, which caused a mild effect compared to Al3+. This interesting feature demonstrated that compound L1 can serve as a selective “naked-eye” chemosensor for Al3+.
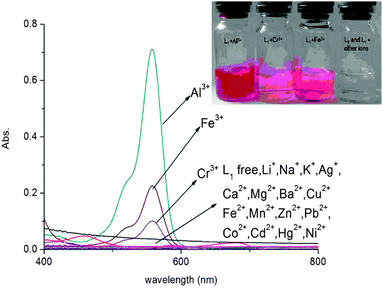 |
| | Fig. 2 Absorbance spectra of L1 (10 μM) in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution in the presence of 10 equiv. of various species. Inset: the photos of L1 with different metal ions in CH3CN/H2O (95 5, v/v) solution in the presence of 10 equiv. of various species. Inset: the photos of L1 with different metal ions in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution. 5, v/v) solution. | |
To further investigate the interaction of Al3+ and L1, an ultraviolet titration experiment was carried out (Fig. 3). The Al3+ binding stoichiometry of L1 can be determined from titration and the Job plot.28 A plot of [Al3+]/{[Al3+] + [L1]} versus the molar fraction of Al3+ is provided in Fig. 4. The absorbance reached a maximum when the ratio was 0.5, indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of Al3+ to L1 in the complex.
1 stoichiometry of Al3+ to L1 in the complex.
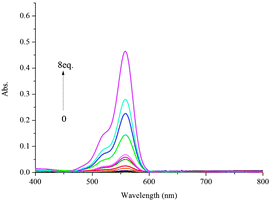 |
| | Fig. 3 Absorbance spectra of L1 (10 μM) in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) upon addition of different amounts of Al3+ ions. 5, v/v) upon addition of different amounts of Al3+ ions. | |
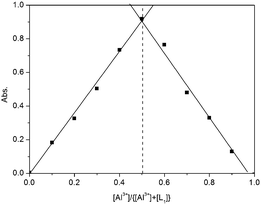 |
| | Fig. 4 Job's plots of the complexation between L1 and Al3+. The total concentration of L1 + Al3+ was kept constant at 100 μM. | |
Fluorescence spectral responses of L1
As shown in Fig. 5, L1 exhibited a very weak fluorescence in the absence of metal ions. When 10 equiv. Al3+ was introduced into a 10 μM solution of L1 in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v), a remarkable enhancement of fluorescence spectra was observed. The fluorescence enhancement of Al3+ to compound L1 was as high as 190-fold. Under the same conditions, a mild fluorescence enhancement factor was also detected for Fe3+ and Cr3+, but Zn2+, Mg2+, Ca2+, Cd2+, Cu2+, Pb2+, Hg2+, Ba2+, Ni2+, Fe2+, Mn2+, K+, Li+, Ag+, Co2+ and Na+ showed no obvious changes in the fluorescence intensity and color. From fluorescence spectra of L2 and L3, we could know Fe3+ and Cr3+ had more interference (Fig. 6 and 7). Probe L1 had better fluorescence properties. It may benefit from the strong electron withdrawing group. Moreover, we also confirmed the competitive experiments that the background metal ions showed very low interference with the detection of Al3+ in water solution (Fig. 8). Generally, the detection limit of metal ions is needed for a fluorescent sensor. Under optical conditions, the linear response for the fluorescence intensity response of compound L1 was between 8 and 18 μM (Fig. 9), and the detection limit of Al3+ was measured to be 3.98 μM.
5, v/v), a remarkable enhancement of fluorescence spectra was observed. The fluorescence enhancement of Al3+ to compound L1 was as high as 190-fold. Under the same conditions, a mild fluorescence enhancement factor was also detected for Fe3+ and Cr3+, but Zn2+, Mg2+, Ca2+, Cd2+, Cu2+, Pb2+, Hg2+, Ba2+, Ni2+, Fe2+, Mn2+, K+, Li+, Ag+, Co2+ and Na+ showed no obvious changes in the fluorescence intensity and color. From fluorescence spectra of L2 and L3, we could know Fe3+ and Cr3+ had more interference (Fig. 6 and 7). Probe L1 had better fluorescence properties. It may benefit from the strong electron withdrawing group. Moreover, we also confirmed the competitive experiments that the background metal ions showed very low interference with the detection of Al3+ in water solution (Fig. 8). Generally, the detection limit of metal ions is needed for a fluorescent sensor. Under optical conditions, the linear response for the fluorescence intensity response of compound L1 was between 8 and 18 μM (Fig. 9), and the detection limit of Al3+ was measured to be 3.98 μM.
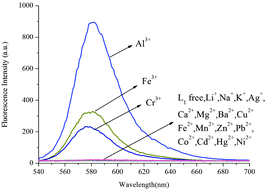 |
| | Fig. 5 Fluorescence spectra of L1 (10 μM) in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm). 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm). | |
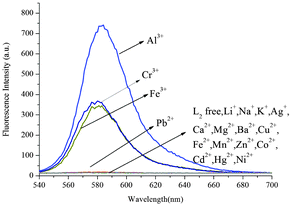 |
| | Fig. 6 Fluorescence spectra of L2 (10 μM) in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm). 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm). | |
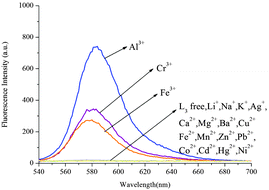 |
| | Fig. 7 Fluorescence spectra of L3 (10 μM) in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm). 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm). | |
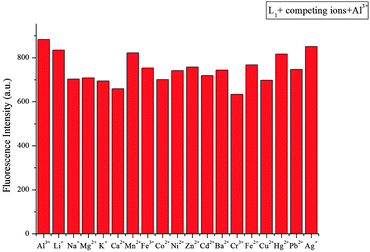 |
| | Fig. 8 Fluorescence intensity (at 582 nm) of L1 upon the addition of 100 μM Al3+ in the presence of 100 μM background metal ions in CH3CN/H2O (95/5, v/v) (λex = 520 nm, slit = 5 nm). | |
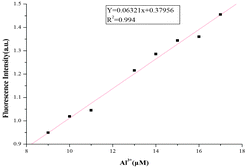 |
| | Fig. 9 Fluorescence intensity (at 582 nm) of compound L1 (10 μM) as a function of the Al3+ concentration in CH3CN/H2O (95/5, v/v) solution (λex = 520 nm, slit = 5 nm). | |
Mechanism
To investigate the Al3+ enhancement mechanism, IR spectra of L1 and L1 + Al3+ were recorded in KBr disks (Fig. S15, ESI†). The peak at 1694 cm−1, which corresponds to the amide carbonyl absorption, disappeared upon the addition of Al3+. This supported the notion that the carbonyl group of L1 is involved in the coordination of metal ions.
In addition, the F−-adding experiments were conducted to examine the reversibility of this reaction and the result is shown in Fig. 10. When F− (3 equiv. of Al3+) was added to the L1 + Al3+ CH3CN/H2O solution, the fluorescence intensity at 582 nm decreased (green line) and further addition of 10 equiv. Al3+ could not recover the fluorescence (blue line). To investigate the fluorescence quenching mechanism, the 1H NMR spectra of the complex of L1 + Al3+ were recorded. As it is seen from Fig. 11, a new single peak was observed at 10.2 ppm when Al3+ was added to L1. Comparing the 1H NMR spectra of p-nitrobenzaldehyde with 1H NMR spectra of the complex of L1 + Al3+, it can be confirmed that the new single peak belongs to the aldehyde proton (Ha) of p-nitrobenzaldehyde (Fig. S12, ESI†). The HR-MS spectra of L1 + Al3+ in CH3CN/H2O (95/5, v/v) were also recorded (Fig. S13, ESI†). A unique peak at m/z 481.2602 corresponding to [L6 + H]+ was clearly observed when 1 equiv. of Al3+ was added to L1, whereas L1 without Al3+ exhibited peaks only at m/z 614.2 which corresponded to [L1 + H]+ (Fig. S3, ESI†). The form of the L6–Al3+ complex was also confirmed by ESI-MS analysis. As shown in Fig. S14 (ESI†), [M + H]+ of L6 appeared at m/z 481.4. When 1 equiv. of Al3+ was added to L1 in H2O/CH3CN, the ESI-MS spectra showed the peak of the L6–Al3+ complex. The signal at m/z 629.4 (calculated value, 629.4) corresponds to [L6 + Al3+ + 2HCOO− + CH3OH]+. Both UV-vis and fluorescence data lead to a significant OFF–ON signal. From the molecular structure and spectral results of L1, an irreversible fluorescent chemodosimeter for Al3+ was constructed as shown in Scheme 2. Firstly, the addition of the Al3+ ion induced a ring opening of the spirolactam of rhodamine. Then, L1–Al was hydrolyzed into p-nitrobenzaldehyde. And it was certified by theoretical calculations (the data are supplied in the ESI†). The DFT calculations were performed using the Gaussian 09 program.29 The structures of L5, H2O, L6 and p-nitrobenzaldehyde were optimized at the B3LYP30–32/6-31G(d) level in acetonitrile solvent, using the integral equation formalism polarizable continuum model (IEF-PCM).33,34 The calculated results demonstrate that the free energy of L6 and p-nitrobenzaldehyde is 14.7 kcal mol−1 lower than those of L5 and H2O, indicating that this hydrolysis step is an exothermic process. So in the reaction, L5 tends to hydrolyze to L6 (Fig. 12). From Fig. 5, we know that both Cr3+ and Fe3+ can induce the moderate fluorescence enhancements to L1. The mechanism of L1 with Fe3+ and Cr3+ was thought to be similar to that of L1 with Al3+. 1H NMR spectra of the complex of L1 + Fe3+ and L1 + Cr3+ were recorded (Fig. S16 and S18, ESI†). The same new single peak was observed at 10.179 ppm and 10.138 ppm, respectively. The ESI mass spectra of L1 + Fe3+ and L1 + Cr3+ in CH3CN/H2O (95/5, v/v) were recorded (Fig. S17 and S19, ESI†) and a similar hydrolysis product was found. A kind of Fe3+-induced Schiff base hydrolysis mechanism has been reported by Kim and coworkers.35
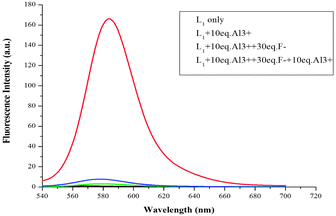 |
| | Fig. 10 Fluorescence intensity (at 582 nm) of L1 (10 μM) as a function of Al3+ concentration in CH3CN/H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solutions (1) baseline: 10 μM L1 only; (2) red line: 10 μM L1 with 10 equiv. Al3+; (3) green line: 10 μM L1 with 10 equiv. Al3+ and then addition of 30 equiv. F−; (4) blue line: 10 μM L1 with 10 equiv. Al3+ and 30 equiv. F− then addition of 10 equiv. Al3+ (λex = 520 nm, slit = 5 nm). 5, v/v) solutions (1) baseline: 10 μM L1 only; (2) red line: 10 μM L1 with 10 equiv. Al3+; (3) green line: 10 μM L1 with 10 equiv. Al3+ and then addition of 30 equiv. F−; (4) blue line: 10 μM L1 with 10 equiv. Al3+ and 30 equiv. F− then addition of 10 equiv. Al3+ (λex = 520 nm, slit = 5 nm). | |
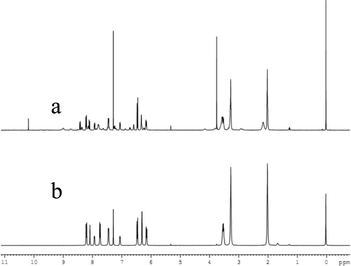 |
| | Fig. 11
1H NMR spectra of L1 + Al3+ (a) and L1 (b) in CDCl3. | |
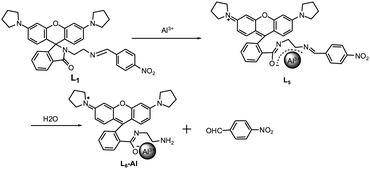 |
| | Scheme 2 Possible sensing mechanism of L1 with Al3+. | |
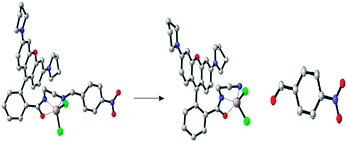 |
| | Fig. 12 Calculated energy-minimized structure of L5, L6 and p-nitrobenzaldehyde (gray: C atoms; blue: N atoms; red: O atoms; green: Cl−; and brown: Al3+). | |
In order to investigate the influence of different acid concentrations on the spectra of L1 and find a suitable pH span in which L1 can selectively detect Al3+ efficiently, the acid titration experiments were performed. The addition of Al3+ led to the fluorescence enhancement over a wide pH range (1.0–8.0), which is attributed to opening of the rhodamine spirolactam structure (Fig. S20, ESI†). Moreover, a time course of the fluorescence response of L1 upon addition of Al3+ is shown in Fig. S21 (ESI†). The kinetics of fluorescence enhancement at 593 nm by the newly developed fluorescent probe was recorded. It indicated that this reaction between L1 and Al3+ was slow. And, the recognizing event of L1 with Fe3+ could be completed in 80 minutes (Fig. S22, ESI†). The chemodosimeters L2 and L3 show similar properties as L1 (see Fig. S23–S34, ESI†). The detection limits of L2 and L3 were measured to be 32 μM (Fig. S27, ESI†) and 49 μM (Fig. S34, ESI†). The three chemodosimeters exhibit irreversible, selective and sensitive recognition toward Al3+ over other metal ions. The colorimetric and fluorometric responses of the sensors L1–L3 and Al3+ can also be conveniently detected by the naked eye. The fluorescence enhancement of Al3+ to L1, L2 and L3 is as high as 190, 120 and 60-fold, respectively. These indicate that the differences among the three chemodosimeters' structures have great influence in their recognition toward Al3+. The strong electron withdrawing group on the benzene ring results in the Schiff base having a good affinity toward Al3+.
Many fluorescent sensors for Al3+ detection could only perform in solution, which would limit their applications under special circumstances such as on-site detection in situ. To demonstrate the practical application of our sensor, we prepared the test papers of sensor L1. It was easily prepared by immersing a filter paper into the solution of L1 in CH2Cl2 (1 mM) and then drying in air. Next, in different Al3+ concentration solutions (0, 1.0 × 10−4 M, 1.0 × 10−3 M, and 1.0 × 10−2 M), these strips were immersed for 5 s and taken out of the solution. As depicted in Fig. 13, the color of the test paper changed from colorless to purple and deepened gradually with the increase of the Al3+ concentration. These paper-made test kits may be used as simple tools for detecting Al3+ in environmental samples. To validate their practicality in real environmental samples, we employed probe L1 in a standard addition method36–38 to determine Al3+ concentrations in water samples from Jinshui river (in Zhengzhou, Henan province, China), no fluorescence enhancement was observed. When the water samples were spiked with different Al3+ concentrations (25 μM, 50 μM, and 100 μM) and measured using the current methods, Al3+ recoveries were about 20% (Table S1, ESI†).
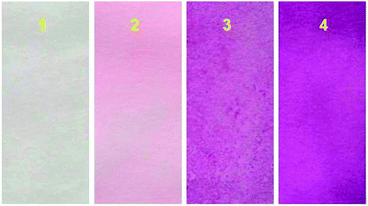 |
| | Fig. 13 Photographs of the test kits with L1 for detecting Al3+ in (CH3CN/H2O = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution with different concentrations: (1) 0; (2) 1.0 × 10−4 M; (3) 1.0 × 10−3 M; (4) 1.0 × 10−2 M. 5, v/v) solution with different concentrations: (1) 0; (2) 1.0 × 10−4 M; (3) 1.0 × 10−3 M; (4) 1.0 × 10−2 M. | |
Conclusions
In summary, we synthesized three fluorescent chemodosimeters L1, L2 and L3. The colorimetric and fluorescence response to Al3+ can be conveniently detected even by the naked eye, which provides a facile method for visual detection of Al3+. Theoretical calculations indicated that the electronic distribution of the N atom of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N is −0.436, −0.46 and −0.464, respectively (log file). Owing to the strong electron withdrawing group on benzene, the Schiff base L1 became stable, which was favorable for high sensitivity for the detection of Al3+. As we know, higher electronegativity of the N atom of C
N is −0.436, −0.46 and −0.464, respectively (log file). Owing to the strong electron withdrawing group on benzene, the Schiff base L1 became stable, which was favorable for high sensitivity for the detection of Al3+. As we know, higher electronegativity of the N atom of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of the Schiff base is easier to hydrolyze. Furthermore, the mechanism of fluorescence was found to be the aluminum complexation with rhodamine and subsequent Al3+-promoted hydrolysis of the Schiff base. Also, iron and chromium complexation had the same hydrolysis mechanism. The simple and convenient test paper may provide an easy way to detect Al3+ in our daily life.
N of the Schiff base is easier to hydrolyze. Furthermore, the mechanism of fluorescence was found to be the aluminum complexation with rhodamine and subsequent Al3+-promoted hydrolysis of the Schiff base. Also, iron and chromium complexation had the same hydrolysis mechanism. The simple and convenient test paper may provide an easy way to detect Al3+ in our daily life.
Acknowledgements
This work was financially supported by the National Science Foundation of China (No. 21375113) and the Program for New Century Excellent Talents in University (NCET-11-0950).
Notes and references
- T. P. Flaten and M. degard, Food Chem. Toxicol., 1988, 26, 959 CrossRef CAS PubMed.
- J. Ren and H. Tian, Sensors, 2007, 7, 3166 CrossRef CAS.
- R. A. Yokel, Neurotoxicology, 2000, 21, 813 CAS.
- G. Muller, V. Bernuzzi, D. Desor, M. F. Hutin, D. Burnel and P. R. Lehr, Teratology, 1990, 42, 253 CrossRef CAS PubMed.
- J. Barceló and C. Poschenrieder, Environ. Exp. Bot., 2002, 48, 75 CrossRef.
- Z. Krejpcio and R. W. Wojciak, Pol. J. Environ. Stud., 2002, 11, 251 CAS.
- D. P. Perl, D. C. Gajdusek, R. M. Garruto, R. T. Yanagihara and C. J. Gibbs, Science, 1982, 217, 1053 CAS.
- D. P. Perl and A. R. Brody, Science, 1980, 208, 297 CAS.
- Y. Chen, K. Y. Han and Y. Liu, Bioorg. Med. Chem., 2007, 15, 4537 CrossRef CAS PubMed.
- R. Partha, D. Koushik, M. Mario, R. Jagnyeswar and B. Pradyot, Inorg. Chem., 2007, 46, 6405 CrossRef PubMed.
- M. Royzen, A. Durandin, V. G. Young, N. E. Geacintov and J. W. Canary, J. Am. Chem. Soc., 2006, 128, 3854 CrossRef CAS PubMed.
- A. Banerjee, A. Sahana, S. Das, S. Lohar, S. Guha, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Analyst, 2012, 137, 2166 RSC.
- H. Y. Lee, K. M. K. Swamy, J. Y. Jung, G. Kim and J. Yoon, Sens. Actuators, B, 2013, 182, 530 CrossRef CAS.
- S. Sen, T. Mukherjee, B. Chattopadhyay, A. Moirangthem, A. Basu, J. Marekd and P. Chattopadhyay, Analyst, 2012, 137, 3975 RSC.
- R. Patil, A. Moirangthem, R. Butcher, N. Singh, A. Basu, K. Tayade, U. Fegade, D. Hundiwale and A. Kuwar, Dalton Trans., 2014, 43, 2895 RSC.
- M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC.
- S. Das, M. Dutta and D. Das, Anal. Methods, 2013, 5, 6262 RSC.
- C. Y. Li, Y. Zhou, Y. F. Li, C. X. Zou and X. F. Kong, Sens. Actuators, B, 2013, 186, 360 CrossRef CAS.
- Y. S. Mi, Z. Cao, Y. T. Chena, S. Long, Q. F. Xie, D. M. Liang, W. P. Zhu and J. N. Xiang, Sens. Actuators, B, 2014, 192, 164 CrossRef CAS.
- S. B. Maity and P. K. Bharadwaj, Inorg. Chem., 2013, 52, 1161 CrossRef CAS PubMed.
- X. X. Fang, S. F. Zhang, G. Y. Zhao, W. W. Zhang, J. W. Xu, A. M. Ren, C. Q. Wu and W. Yang, Dyes Pigm., 2014, 101, 58 CrossRef CAS.
- A. Sahana, A. Banerjee, S. Lohar, B. Sarkar, S. K. Mukhopadhyay and D. Das, Inorg. Chem., 2013, 52, 3627 CrossRef CAS PubMed.
- C. Lohani, J. Kim, S. Chung, J. Yoon and K. Lee, Analyst, 2010, 135, 2079 RSC.
- Z. Li, Q. Hu, C. Li, J. Dou, J. Cao, W. Chen and Q. Zhu, Tetrahedron Lett., 2014, 55, 1258 CrossRef CAS.
- L. Y. Wang, L. L. Yang and D. R. Cao, Sens. Actuators, B, 2014, 202, 949 CrossRef CAS.
- K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Dalton Trans., 2013, 42, 13311 RSC.
- J. H. Wang, D. Zhang, Y. Q. Liu, P. G. Ding, C. C. Wang, Y. Ye and Y. F. Zhao, Sens. Actuators, B, 2014, 191, 344 CrossRef CAS.
- C. Y. Huang, Methods Enzymol., 1982, 87, 509 CAS.
-
G. W. Trucks, M. J. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Revision C.01, 2010 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater., 1988, 37, 785 CrossRef CAS.
- B. Miehlich, A. Savin, H. Stoll and H. Preuss, Chem. Phys. Lett., 1989, 157, 200 CrossRef CAS.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995 CrossRef CAS.
- B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 106, 5151 CrossRef CAS.
- M. H. Lee, T. V. Giap, S. H. Kim, Y. H. Lee, C. H. Kang and J. S. Kim, Chem. Commun., 2010, 46, 1407 RSC.
- Z. Wang, D. M. Han, W. P. Jia, Q. Z. Zhou and W. P. Deng, Anal. Chem., 2012, 84, 4915–4920 CrossRef CAS PubMed.
- J. V. Ros-Lis, B. Garcia, D. Jimenez, R. Martinez-Manez, F. Sancenon, J. Soto, F. Gonzalvo and M. C. Valldecabres, J. Am. Chem. Soc., 2004, 126, 4064–4065 CrossRef CAS PubMed.
- J. Li, C. F. Zhang, S. H. Yang, W. C. Yang and G. F. Yang, Anal. Chem., 2014, 86, 3037–3042 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nj01357c |
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution with appropriate amounts of metal ions. Solutions were shaken for 120 min before measuring the absorption and fluorescence in order to make the metal ions chelate with the sensors sufficiently.
5, v/v) solution with appropriate amounts of metal ions. Solutions were shaken for 120 min before measuring the absorption and fluorescence in order to make the metal ions chelate with the sensors sufficiently.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of Al3+ to L1 in the complex.
1 stoichiometry of Al3+ to L1 in the complex.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) upon addition of different amounts of Al3+ ions.
5, v/v) upon addition of different amounts of Al3+ ions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v), a remarkable enhancement of fluorescence spectra was observed. The fluorescence enhancement of Al3+ to compound L1 was as high as 190-fold. Under the same conditions, a mild fluorescence enhancement factor was also detected for Fe3+ and Cr3+, but Zn2+, Mg2+, Ca2+, Cd2+, Cu2+, Pb2+, Hg2+, Ba2+, Ni2+, Fe2+, Mn2+, K+, Li+, Ag+, Co2+ and Na+ showed no obvious changes in the fluorescence intensity and color. From fluorescence spectra of L2 and L3, we could know Fe3+ and Cr3+ had more interference (Fig. 6 and 7). Probe L1 had better fluorescence properties. It may benefit from the strong electron withdrawing group. Moreover, we also confirmed the competitive experiments that the background metal ions showed very low interference with the detection of Al3+ in water solution (Fig. 8). Generally, the detection limit of metal ions is needed for a fluorescent sensor. Under optical conditions, the linear response for the fluorescence intensity response of compound L1 was between 8 and 18 μM (Fig. 9), and the detection limit of Al3+ was measured to be 3.98 μM.
5, v/v), a remarkable enhancement of fluorescence spectra was observed. The fluorescence enhancement of Al3+ to compound L1 was as high as 190-fold. Under the same conditions, a mild fluorescence enhancement factor was also detected for Fe3+ and Cr3+, but Zn2+, Mg2+, Ca2+, Cd2+, Cu2+, Pb2+, Hg2+, Ba2+, Ni2+, Fe2+, Mn2+, K+, Li+, Ag+, Co2+ and Na+ showed no obvious changes in the fluorescence intensity and color. From fluorescence spectra of L2 and L3, we could know Fe3+ and Cr3+ had more interference (Fig. 6 and 7). Probe L1 had better fluorescence properties. It may benefit from the strong electron withdrawing group. Moreover, we also confirmed the competitive experiments that the background metal ions showed very low interference with the detection of Al3+ in water solution (Fig. 8). Generally, the detection limit of metal ions is needed for a fluorescent sensor. Under optical conditions, the linear response for the fluorescence intensity response of compound L1 was between 8 and 18 μM (Fig. 9), and the detection limit of Al3+ was measured to be 3.98 μM.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm).
5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm).
5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm).
5, v/v) in the presence of 10 equiv. of various metal ions (λex = 520 nm, slit = 5 nm).



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution with different concentrations: (1) 0; (2) 1.0 × 10−4 M; (3) 1.0 × 10−3 M; (4) 1.0 × 10−2 M.
5, v/v) solution with different concentrations: (1) 0; (2) 1.0 × 10−4 M; (3) 1.0 × 10−3 M; (4) 1.0 × 10−2 M.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N is −0.436, −0.46 and −0.464, respectively (log file). Owing to the strong electron withdrawing group on benzene, the Schiff base L1 became stable, which was favorable for high sensitivity for the detection of Al3+. As we know, higher electronegativity of the N atom of C
N is −0.436, −0.46 and −0.464, respectively (log file). Owing to the strong electron withdrawing group on benzene, the Schiff base L1 became stable, which was favorable for high sensitivity for the detection of Al3+. As we know, higher electronegativity of the N atom of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of the Schiff base is easier to hydrolyze. Furthermore, the mechanism of fluorescence was found to be the aluminum complexation with rhodamine and subsequent Al3+-promoted hydrolysis of the Schiff base. Also, iron and chromium complexation had the same hydrolysis mechanism. The simple and convenient test paper may provide an easy way to detect Al3+ in our daily life.
N of the Schiff base is easier to hydrolyze. Furthermore, the mechanism of fluorescence was found to be the aluminum complexation with rhodamine and subsequent Al3+-promoted hydrolysis of the Schiff base. Also, iron and chromium complexation had the same hydrolysis mechanism. The simple and convenient test paper may provide an easy way to detect Al3+ in our daily life.






