Controllable fabrication of multifunctional 1D Ag-based coordination polymer@PVP nanowires†
Bo
Lv
a,
Xiaobo
Shi
b,
Xiaoyan
Ma
c,
Zhiyang
Zhang
c and
Kuaibing
Wang
*a
aDepartment of Chemistry, College of Science, Nanjing Agricultural University, Nanjing 210095, P. R. China. E-mail: wangkb@njau.edu.cn
bCollege of Agriculture, Nanjing Agricultural University, Nanjing 210095, P. R. China
cState Key Laboratory of Coordination Chemistry, Coordination Chemistry Institute, Nanjing National Laboratory of Microstructures, Nanjing University, Nanjing 210093, P. R. China
First published on 14th October 2014
Abstract
Wire-like coordination polymer architectures, coupled with polyvinylpyrrolidone (PVP) additive, have been synthesized under mild conditions. The morphology of the final coordination polymer was dependent on the synthetic parameters, such as solvents and the amount of PVP. A possible assembly mechanism for the composite nanowires, based on several characterization methods, has been proposed to interpret the growth process. In addition, the newly synthesized Ag-based polymer particles may act as novel antimicrobial agents and metal-based anticancer drugs in the future owing to their potent antibacterial and in vitro anticancer activities against the four selected cancer lines MCF-7, HeLa, H1299 and A549.
Introduction
In the past decade, significant efforts have been focused on developing new methods for the controlled fabrication of one dimensional (1D) nanoscale structures, such as wires, belts, tubes, and fibers, due to their unique applications in optics, electronics, sensors and magnetic devices.1–3 Coordination polymer nanoparticles (CPN) are an important emerging class of 1D nanomaterials with the intriguing prospect of generating controllable morphologies and sizes by the elaborate selection of both metal ions and organic building blocks.4–7 Furthermore, the manipulation of particle size and morphology plays a vital role in controlling the resulting chemical and physical properties. To date, the synthetic approaches for 1D CPN have received considerable attention and progress has been made in layer-by-layer method and microfluidic strategy.8–12 However, regulating and guiding the assembly process between metal ions and organic molecules to novel 1D coordination polymer structures at the nanometer length scale still remains a challenge.The relatively weak interactions of the coordination bonds dominate the hierarchical self-assembly process, which distinguishes this class of molecular-based materials from dense inorganic materials, such as quantum dots and semiconductors.13–15 Thus, it is important to control the interactions between metal ions and organic ligands (so-called “coordination equilibria”) when regulating the corresponding synthetic parameters to vary the final morphology, size and crystallinity. In this regard, Kitagawa and co-workers created a competitive environment that regulates the rate of framework extension and crystal growth by simply introducing a capping reagent to modulate the coordination equilibria.16 Herein, inspired by the preliminary work done by Kitagawa, polyvinylpyrrolidone (PVP) has been documented as templating, capping and competitive additive for the synthesis of 1D Ag-based nanowires. In addition, we describe the utilization of the resulting CPN for its application in antibacterial and in vitro anticancer fields.
Experimental
Preparation of Ag-based CPN
The organic building blocks 1,3,5-benzenetricarboxylic acid (H3BTC) and NaOH with the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were dissolved in deionized water to form 0.1 M Na3BTC aqueous solution. In a typical synthesis of nanosheets (CPN-2), 0.1 M Na3BTC (0.2 mL) aqueous solution was dropwise introduced into 0.1 M AgNO3 (0.6 mL) aqueous solution in a water/ethanol system (10/10, v
3 were dissolved in deionized water to form 0.1 M Na3BTC aqueous solution. In a typical synthesis of nanosheets (CPN-2), 0.1 M Na3BTC (0.2 mL) aqueous solution was dropwise introduced into 0.1 M AgNO3 (0.6 mL) aqueous solution in a water/ethanol system (10/10, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) with vigorous stirring, which immediately resulted in a large amount of white precipitate. After stirring for 20 min, the precipitate was collected by centrifugation, and washed three times with ethanol and water. In addition, in the presence of polyvinylpyrrolidone (PVP K30, M ≈ 58
v) with vigorous stirring, which immediately resulted in a large amount of white precipitate. After stirring for 20 min, the precipitate was collected by centrifugation, and washed three times with ethanol and water. In addition, in the presence of polyvinylpyrrolidone (PVP K30, M ≈ 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 0.1 g), the same reactant concentration and treating procedure, as used for CPN-2, can be used to obtain CPN-1 nanowires. The other shaped Ag-based samples were generated by adjusting the synthetic parameters, such as solvents, the amount of sodium dodecyl sulfonate (SDS) and PVP. Notably: (i) the surfactant was first dissolved in the solvent with Ag metal ions, and then the organic linkers were slowly added into the solution; (ii) all the reactions were carried out under the dark. The resulting products were dried under vacuum at 25 °C for 4 h, and were further characterized. Elemental analysis (EA) for CPN-1: C, 19.28%; H, 3.316%; N, 1.106%. EA for CPN-2: C, 17.67%; H, 1.816%; N, 0.056%.
000, 0.1 g), the same reactant concentration and treating procedure, as used for CPN-2, can be used to obtain CPN-1 nanowires. The other shaped Ag-based samples were generated by adjusting the synthetic parameters, such as solvents, the amount of sodium dodecyl sulfonate (SDS) and PVP. Notably: (i) the surfactant was first dissolved in the solvent with Ag metal ions, and then the organic linkers were slowly added into the solution; (ii) all the reactions were carried out under the dark. The resulting products were dried under vacuum at 25 °C for 4 h, and were further characterized. Elemental analysis (EA) for CPN-1: C, 19.28%; H, 3.316%; N, 1.106%. EA for CPN-2: C, 17.67%; H, 1.816%; N, 0.056%.
Antibacterial test
Cultures of Escherichia coli (E. coli) and Bacillus subtilis were grown in LB broth. Fresh overnight culture (6 h for E. coli and 12 h for B. subtilis at 37 °C, respectively) was inoculated into LB broth containing 50 μg mL−1 of a sample and incubated at 37 °C for 12 h. On the compounds (at concentration of 50 μg mL−1) showing no turbidity after incubation, a more extensive test was performed. The minimum inhibitory concentrations (MIC, μg mL−1) was measured using a series of glass test-tubes containing the compound at the concentration of 50, 4, 2, 1, 0.5, and 0.25 μg mL−1. Each tube was inoculated with exponential-growth-phase organisms (E. coli or B. subtilis) to a 1% concentration and incubated at 37 °C for 6 h and 12 h, respectively. The optical density (OD) at 600 nm was measured before and after the incubation. Broth containing bacteria alone was used as a positive control. Each test was performed in triplicate.Cell viability assay
3 × 104 cancer cells (MCF-7, HeLa, A549 and H1299) per well were seeded in 96-well plates (TPP, St. Louis, MO, USA) in complete medium and maintained at 37 °C in a humidified atmosphere of 5% CO2 and incubated for 12 h before experimental treatments. Then, the cells were treated with various concentrations of CPN-1, CPN-2, AgNO3 and sole 0.5% DMSO–H2O solution for 24 and 48 h. Then, the culture medium was removed and fresh 200 μL of complete medium containing 20 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg mL−1 in PBS) was added and medium was subjected to further 4 h of incubation. After the supernatant had been removed, 150 μL per well of DMSO was added to dissolve the purple formazan crystals. The absorbance was read on Thermo Scientific Varioskan Flash at 600 nm. The non-treated cells (in DMEM) were used as control and the relative cell viability (mean% ± SD, n = 3) was expressed as OD sample/OD control × 100%.General methods
Solvents and all other chemicals were obtained from commercial sources and used as received unless otherwise noted. X-ray powder diffraction (XRD) data were collected on a Bruker D8 Advance instrument using CuKα radiation (λ = 1.54056 Å) at room temperature. The morphology of the as-prepared samples and the corresponding energy dispersive X-ray (EDX) spectroscopy were obtained using a Hitachi S-4800 field-emission scanning electron microscope (FE-SEM). X-ray photoelectron spectroscopy (XPS) was collected on an ESCALab MKII X-ray photoelectron spectrometer using non-monochromatized AlKα X-ray as excitation source. Fourier-transformed infrared (FT-IR) spectra were obtained on a Bruker Vector 22 FT-IR spectrophotometer using KBr pellets. The elemental analyses of C, H, and N were performed on an Elementar Vario MICRO Elemental Analyzer at the Analysis Centre of Nanjing University.Results and discussion
1D Ag-based coordination polymer nanowires (CPN-1) were synthesized by slowly adding a solution of Na3BTC (0.1 M, 0.2 mL) to a solution of AgNO3 (0.1 M, 0.6 mL) in the presence of PVP (0.1 g), and characterized through field-emission scanning electron microscopy images (FE-SEM) as shown in Fig. 1. Fig. 1a revealed the formation of bundle-like products with the approximate length of 15 μm for each bundle, which appears to be tied up at the centre with a rubber band. From the magnification of SEM images, as shown in Fig. 1b–d, both ends consist of a number of nanowires with the average widths of 48 ± 14 nm. In the experimental process, the dispersion of nanowires was very efficient, which can be confirmed from an image obtained after allowing it to stand for 12 h (Fig. 1e). To clarify the effect of the modulator (PVP) on the mechanism of framework construction and crystal growth, the amount of PVP was altered across the range of 0–1 g. Keeping the same reactant concentration in the absence of PVP, a 2D nanosheet product (CPN-2) was generated on a large scale to form another type of white precipitate and its morphology was observed by SEM, as depicted in Fig. 2. The mean length and width of sheets was 9.8 ± 1.4 μm and 1.2 ± 0.4 μm, respectively. Notably, the 2D sheet was very thin with mean thickness of ca. 37 nm. The compared result indicates that PVP, a long-chain and flexible polymer, can act to be a template agent for the fabrication of nanowires. In this set of experiments, the concentration of Na3BTC (0.1 M, 0.2 mL) and AgNO3 (0.1 M, 0.6 mL) are defined to be standard concentrations. | ||
| Fig. 1 (a–d) SEM images of wire morphology for CPN-1. (e) The image of CPN-1 after allowing it to stand at room temperature for 12 h. | ||
 | ||
| Fig. 2 (a–d) SEM images of sheet morphology for CPN-2 in the absence of PVP. (e) SEM image of wires with viscosity morphology in 0.5 g of PVP. (f) SEM image of dumbbell morphology in 1 g of PVP. | ||
When the amount of PVP was increased to 0.5 g or 1 g while maintaining the rest of the conditions, there was no precipitate formed and the corresponding concentration ratios of the reactant were increased to 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 and 0.4
0.9 and 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. This indicated that Ag metal ions were wrapped in PVP additive, and thus impeded the coordination interactions with organic linkers. Consequently, nanowires with larger viscosity and smaller sizes (mean length of 8 μm) were formed at a concentration ratio of 0.3
1.2. This indicated that Ag metal ions were wrapped in PVP additive, and thus impeded the coordination interactions with organic linkers. Consequently, nanowires with larger viscosity and smaller sizes (mean length of 8 μm) were formed at a concentration ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9, as demonstrated in Fig. 2e. However, with the amount of PVP increasing to 1 g, the morphology was significantly altered from nanowires to dumbbell-like products (Fig. 2f). Therefore, the proper increase in the amount of PVP significantly decelerated the rate of crystal growth and facilitated the formation of nanowires (in the range of 0.1–0.5 g in this study).
0.9, as demonstrated in Fig. 2e. However, with the amount of PVP increasing to 1 g, the morphology was significantly altered from nanowires to dumbbell-like products (Fig. 2f). Therefore, the proper increase in the amount of PVP significantly decelerated the rate of crystal growth and facilitated the formation of nanowires (in the range of 0.1–0.5 g in this study).
The other synthetic parameters, such as solvents and the amount of SDS are also documented to emphasize the effect of PVP. Under the standard concentrations in the presence of PVP (0.1 g), varying the solvent from a water–ethanol system to pure water (20 mL), there was no formation of any other particles, with the exception of adding the concentration ratio to 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8. The resulting morphology was the same as the CPN-1 nanowires, as demonstrated in Fig. S1 (see ESI†). This result reveals that different solvents can also decrease the rate of framework extension, and thus influence the final reactant concentration used. In addition, upon replacing PVP with SDS (0.1 g) and maintaining the reactant at the standard concentration, hackly belt-like products were obtained with an average length and width of about 20 μm and 1 μm, respectively (Fig. S2, ESI†).
1.8. The resulting morphology was the same as the CPN-1 nanowires, as demonstrated in Fig. S1 (see ESI†). This result reveals that different solvents can also decrease the rate of framework extension, and thus influence the final reactant concentration used. In addition, upon replacing PVP with SDS (0.1 g) and maintaining the reactant at the standard concentration, hackly belt-like products were obtained with an average length and width of about 20 μm and 1 μm, respectively (Fig. S2, ESI†).
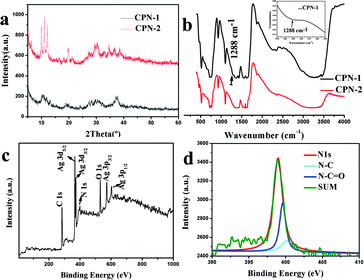
The structures of the coordination polymer species of CPN-1 and CPN-2 are supported by X-ray powder diffraction (XRD) results, as shown in Fig. 3a. The patterns of CPN-2 had sharp diffraction peaks for the nanosheets, in contrast to the broader diffraction peaks for CPN-2 nanowires (Fig. 3a). This result is in agreement with the experimental phenomenon and suggests that CPN-1 nanowires are wrapped in PVP additive to form CPN-1@PVP composite, thus affecting their resulting crystallinity, which can be confirmed by infrared spectroscopy (IR) (Fig. 3b), energy dispersive X-ray (EDX) spectroscopy and elemental analysis (EA) (Fig. S3, ESI† and Experimental section). The CO stretching frequency at 1695 cm−1 for uncoordinated H3BTC shifted to around 1610 cm−1, illustrating the complete deprotonation of the carboxylate group after the formation of coordination polymers. As illustrated in Fig. 3b, the weak stretching frequency at 1288 cm−1 for CPN-1 may be assigned to –NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, revealing the existence of PVP because of the accordance with the characteristic peak of amide group.17 In addition, EDX confirms that the CPN-1 nanowires are composed of Ag, C, N and O, in agreement with the results of EA. For further proving the existence of PVP, X-ray photoelectron spectroscopy (XPS) was also performed, as illustrated in Fig. 3c and d. Within the survey region (0–1000 eV), carbon, oxygen, silver and nitrogen species were detected (Fig. 3c). The deconvolution of the N 1s peak revealed the presence of nitrogen species at 398.9 eV (Fig. 3d). There were a large amount of functional groups in the PVP additive, such as N–C or N–C
O, revealing the existence of PVP because of the accordance with the characteristic peak of amide group.17 In addition, EDX confirms that the CPN-1 nanowires are composed of Ag, C, N and O, in agreement with the results of EA. For further proving the existence of PVP, X-ray photoelectron spectroscopy (XPS) was also performed, as illustrated in Fig. 3c and d. Within the survey region (0–1000 eV), carbon, oxygen, silver and nitrogen species were detected (Fig. 3c). The deconvolution of the N 1s peak revealed the presence of nitrogen species at 398.9 eV (Fig. 3d). There were a large amount of functional groups in the PVP additive, such as N–C or N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, which covalently bond onto the Ag-based coordination polymers during self-assembling process to form CPN-1@PVP composite. The chemical composition of other Ag-based samples characterized by IR, XRD and EA are illustrated in Fig. S4 and Table S1 (ESI†). The results show that changing the corresponding synthetic parameters did not change the coordination polymer nature.
O groups, which covalently bond onto the Ag-based coordination polymers during self-assembling process to form CPN-1@PVP composite. The chemical composition of other Ag-based samples characterized by IR, XRD and EA are illustrated in Fig. S4 and Table S1 (ESI†). The results show that changing the corresponding synthetic parameters did not change the coordination polymer nature.
On the basis of these results, we propose a growth mechanism for the nanowires of CPN-1 (Scheme 1). PVP, a water-soluble polymer, often forms random coils in solution. In the presence of Ag metal ions, O atoms in PVP chain can coordinate with it to form cross-linking PVP chains and there is electrostatic repulsion between them. The introduction of intermolecular or intramolecular Ag crosslinks improves the rigidity of the PVP chains, and thus changes the mobility of the chains. The mobility makes the coordination reactions of Ag metal ions with organic linker to occur, further enhancing the stability of wires-like CPN-1@PVP bundles. As a result, proper rigidity and electrostatic repulsion between PVP additives play important roles in generating composite wires.
This mechanism is also supported by the observation that the different antibacterial effects were quantitatively evaluated against both Gram-negative E. coli and Gram-positive B. subtilis by studying the bacterial growth kinetics in LB liquid media. The bacterial proliferation was monitored by investigating optical density at 600 nm (OD600) based on the turbidity of the cell suspension within 12 h (Fig. 4). Remarkably, the growth of both Gram-negative and Gram-positive bacteria was completely inhibited during the entire 12 h culture period. It is worth noting the following facts: (i) CPN-1 and CPN-2 had different antibacterial activities against Gram-negative (Fig. 4a and b) and Gram-positive (Fig. 4c and d) after 6 or 12 h of incubation. The result may indicate that the capping and coordinating effect of PVP additive restrains the diffusion of Ag metal ions from the composite nanowires, accordingly leading to different antimicrobial activity from CPN-2 or AgNO3. After 12 h of incubation, the MIC (minimum inhibitory concentration) for CPN-1 nanowires, defined as the lowest concentration of an antimicrobial material that can inhibit bacterial growth, was 4 μg mL−1 for B. subtilis and 2 μg mL−1 for E. coli. These high-efficiency and rapid antibacterial activities were close to those of compared sample (AgNO3) and other reported Ag-based materials,18 which is particularly important for practical applications. The antibacterial activity for CPN-1 and CPN-2 was also investigated through observing the turbidity of the cell suspension, as vividly depicted in Fig. S5 and S6 (ESI†). We found that the samples, whether CPN-1 nanowires or CPN-2 nanosheets at a concentration of 2 μg mL−1, could completely inhibit bacterial growth for both Gram-negative and Gram-positive bacteria, which were in agreement with the data of growth kinetics. It is noteworthy that the antibacterial activities of the two as-synthesized compounds are significantly better than those of reported Ag-based complexes with non-fluorinated ligands as well as Ag nanoparticles, and close to those of Ag-based compounds with fluorinated ligands.19–21
Subsequently, the newly synthesized CPN-1, CPN-2 and the control sample, AgNO3, were first evaluated for their cytotoxic activities toward HeLa cancer cells. Cytotoxicity was investigated using MTT tests after 24 and 48 hours of treatment with the increasing concentrations of the tested compounds (Fig. 5). The samples of CPN-1 and CPN-2 lead to a dose-dependent cytotoxicity in the range of 1–6 μg mL−1. After 24 h of treatment, the cell viabilities of CPN-1 and AgNO3 were maintained at around 21.9% and 14.2%, at a concentration of 6 μg mL−1. After 48 h, the cell viabilities of CPN-1 and AgNO3 remain at the same value of 6.4%. However, the cell viabilities for CPN-2, after 24 h and 48 h of incubation, show the similar results as AgNO3 product and maintain about 14.2% and 6.4%, respectively. In addition, IC50 values of the tested samples after treating for 48 h in the MTT assay, calculated from the dose-viability curves, are 1.77 ± 0.08 for CPN-1, 1.51 ± 0.06 for CPN-2, and 2.02 ± 0.15 μg mL−1 for AgNO3 (Table 1). Moreover, in vitro antitumor activities.
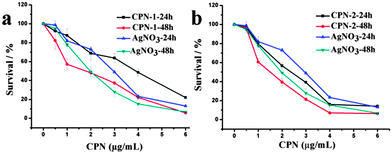 | ||
| Fig. 5 (a and b) The viabilities of HeLa cells incubated with AgNO3 and the as-synthesized samples at the increasing concentrations for different time periods. | ||
| IC50 | ||||||
|---|---|---|---|---|---|---|
| CPN-1 | CPN-2 | AgNO3 | ||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| A549 | 3.87 ± 0.19 | 3.49 ± 0.16 | 1.42 ± 0.07 | 1.09 ± 0.09 | 3.74 ± 0.17 | 2.56 ± 0.13 |
| H1299 | 3.65 ± 0.18 | 3.03 ± 0.14 | 1.97 ± 0.10 | 1.59 ± 0.08 | 2.56 ± 0.12 | 2.14 ± 0.11 |
| Hela | 3.88 ± 0.19 | 1.77 ± 0.08 | 2.37 ± 0.11 | 1.51 ± 0.06 | 2.95 ± 0.15 | 2.02 ± 0.15 |
| MCF-7 | 5.37 ± 0.23 | 4.13 ± 0.21 | 4.06 ± 0.21 | 2.58 ± 0.14 | 3.35 ± 0.16 | 2.51 ± 0.13 |
Of the as-prepared CPN against other three cancer cell lines A549, H1299 and MCF-7 were also conducted. The results show that the as-obtained CPN still have good in vitro anticancer activities against tested cancer cells and detailed IC50 values can be seen in Table 1. Although the underlying antitumor mechanism of CPN is still uncertain, these observed results are highly significant as the good anticancer activities of the Ag-based CPN may be attributed to the interaction of CPN with the cell body or the disassociation of Ag ions from CPN. Furthermore, the slightly different antitumor activity between CPN-1 and CPN-2 also indicates the effect of PVP on the resulting properties and confirms our deduction about the architectures of CPN-1 composite wires.
Conclusions
In summary, 1D Ag-based coordination polymer nanowires and 2D nanosheets have been synthesized by adjusting the amount of PVP additive, which can act as both capping and template reagent, and is highly important in fabricating the resulting product, and therefore influencing the final properties. Based on these results, we describe a proper crystal growth mechanism for CPN-1@PVP composite nanowires and verify the deduction through the technical characterization, such as SEM, XRD, IR, EA and XPS. In addition, the as-synthesized CPN exhibit good antibacterial and antitumor properties, allowing them to be viable candidates for potential applications in medical fields. Our research on PVP-assisted approach for 1D coordination polymer nanomaterials is still in progress.Acknowledgements
This work was supported by the Foundation of College of Science (050802001) and the Scientific Research Foundation of Nanjing Agricultural University (050804087).Notes and references
- (a) C. Joachim, J. K. Gimzewski and A. Aviram, Nature, 2000, 408, 541 CrossRef CAS PubMed; (b) Y. Huang, X. F. Duan, Y. Cui, L. J. Lauhon, K. H. Kim and C. M. Lieber, Science, 2001, 294, 1313 CrossRef CAS PubMed.
- (a) F. S. Schoonbeek, J. H. van Esch, B. Wegewijs, D. B. A. Rep, M. P. de Haas, T. M. Klapwijk, R. M. Kellogg and B. L. Feringa, Angew. Chem., Int. Ed., 1999, 38, 1393 CrossRef CAS; (b) D. B. Amabilino and J. Puigmarti-Luis, Soft Mater., 2010, 6, 1605 RSC.
- R. V. Ulijn and A. M. Smith, Chem. Soc. Rev., 2008, 37, 664 RSC.
- (a) A. M. Spokoyny, D. Kim, A. Sumrein and C. A. Mirkin, Chem. Soc. Rev., 2009, 38, 1218 RSC; (b) A. Carné, C. Carbonell, I. Imaz and D. Maspoch, Chem. Soc. Rev., 2011, 40, 291 RSC.
- (a) J. D. Rocca, D. M. Liu and W. B. Lin, Acc. Chem. Res., 2011, 44, 957 CrossRef PubMed; (b) W. Lin, W. J. Rieter and K. M. L. Taylor, Angew. Chem., Int. Ed., 2009, 48, 650 CrossRef CAS PubMed.
- (a) J. K.-H. Hui and M. J. MacLachlan, Coord. Chem. Rev., 2010, 254, 2363 CrossRef CAS PubMed; (b) S. Dang, Q. Liu, K. Liu, Z. Guo, L. Sun, S. Song and H. Zhang, Cryst. Growth Des., 2010, 10, 4662 CrossRef CAS.
- (a) K. B. Wang, Y. X. Yin, C. Y. Li, Z. R. Geng and Z. L. Wang, CrystEngComm, 2011, 13, 6231 RSC; (b) K. B. Wang, X. Y. Ma, Z. Y. Zhang, M. B. Zheng, Z. R. Geng and Z. L. Wang, Chem. – Eur. J., 2013, 19, 7084 CrossRef CAS PubMed.
- I. Imaz, M. Rubio-Martínez, W. J. Saletra, D. B. Amabilino and D. Maspoch, J. Am. Chem. Soc., 2009, 131, 18222 CrossRef CAS PubMed.
- J. Puigmartí-Luis, M. Rubio-Martínez, U. Hartfelder, I. Imaz, D. Maspoch and P. S. Dittrich, J. Am. Chem. Soc., 2011, 133, 4216 CrossRef PubMed.
- (a) S. Diring, S. Furukawa, Y. Takashima, T. Tsuruoka and S. Kitagawa, Chem. Mater., 2010, 22, 4531 CrossRef CAS; (b) Z. Ni and R. I. Masel, J. Am. Chem. Soc., 2006, 128, 12394 CrossRef CAS PubMed; (c) Y.-M. Jeon, G. S. Armatas, D. Kim, M. G. Kanatzidis and C. A. Mirkin, Small, 2009, 5, 46 CrossRef CAS PubMed.
- (a) I. Imaz, M. Rubio-Martínez, L. García-Fernández, F. García, D. Ruiz-Molina, J. Hernando, V. Puntes and D. Maspoch, Chem. Commun., 2010, 46, 4737 RSC; (b) S. Jung and M. Oh, Angew. Chem., Int. Ed., 2008, 47, 2049 CrossRef CAS PubMed.
- (a) W. Cho, Y. H. Lee, H. J. Lee and M. Oh, Adv. Mater., 2011, 23, 1720 CrossRef CAS PubMed; (b) Y.-M. Jeon, G. S. Armatas, J. Heo, M. G. Kanatzidis and C. A. Mirkin, Adv. Mater., 2008, 20, 2105 CrossRef CAS; (c) W. J. Rieter, K. M. L. Taylor, H. An, W. Lin and W. Lin, J. Am. Chem. Soc., 2006, 128, 9024 CrossRef CAS PubMed.
- (a) A. P. Alivisatos, Science, 1996, 271, 933 CAS; (b) X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed.
- H. Usta, A. Facchetti and T. J. Marks, Acc. Chem. Res., 2011, 44, 501 CrossRef CAS PubMed.
- (a) Z. L. Wang and J. H. Song, Science, 2006, 312, 242 CrossRef CAS PubMed; (b) H. Pang, Q. Y. Lu, J. J. Wang, Y. Q. Li and F. Gao, Chem. Commun., 2010, 46, 2010 RSC.
- T. Tsuruoka, S. Furukawa, Y. Takashima, K. Yoshida, S. Isoda and S. Kitagawa, Angew. Chem., Int. Ed., 2009, 48, 4739 CrossRef CAS PubMed.
- T. Ashida, K. Miura, T. Nomoto, S. Yagi, H. Sumida, G. Kutluk, K. Soda, H. Namatame and M. Taniguchi, Surf. Sci., 2005, 601, 3898 CrossRef PubMed.
- M. Lv, S. Su, Y. He, Q. Huang, W. Hu, D. Li, C. Fan and S. T. Lee, Adv. Mater., 2010, 22, 5463 CrossRef CAS PubMed.
- (a) K. Nomiya, S. Takahashi, R. Noguchi, S. Nemoto, T. Takayama and M. Oda, Inorg. Chem., 2000, 39, 3301 CrossRef CAS; (b) K. Nomiya, S. Takahashi and R. Noguchi, J. Chem. Soc., Dalton Trans., 2000, 1343 RSC.
- (a) D. M. Eby, N. M. Schaeublin, K. E. Farrington, S. M. Hussain and G. R. Johnson, ACS Nano, 2009, 3, 984 CrossRef CAS PubMed; (b) J. Jain, S. Arora, J. M. Rajwade, P. Omray, S. Khandelwal and K. M. Paknikar, Mol. Pharmaceutics, 2009, 6, 1388 CrossRef CAS PubMed.
- S. C. Chen, Z. H. Zhang, Q. Chen, L. Q. Wang, J. Xu, M. Y. He, M. Du, X. P. Yang and R. A. Jones, Chem. Commun., 2013, 49, 1270 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images for other Ag-based CPN under different conditions, EDX patterns for CPN-1 and CPN-2, and the images of antibacterial activities. See DOI: 10.1039/c4nj00719k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |