Facile synthesis of Fe3O4 nanoparticles on metal organic framework MIL-101(Cr): characterization and catalytic activity†
Mrinal
Saikia
ab,
Diganta
Bhuyan
a and
Lakshi
Saikia
 *ab
*ab
aMaterials Science Division, CSIR-North East Institute of Science and Technology, Jorhat, Assam, India. E-mail: lakshi_saikia@yahoo.com; Fax: +91 0376 2370011; Tel: +91 0376 2370081
bAcademy of Scientific and Innovative Research, New Delhi, India
First published on 25th September 2014
Abstract
Fe3O4 nanoparticles can be effectively incorporated into the matrix of MIL-101(Cr) to fabricate a Fe3O4@MIL-101 magnetite nanocomposite which behaves as a magnetic nanocatalyst for the solvent free oxidation of benzyl alcohol in the presence of TBHP. The composite material is magnetically separable and can be reused several times.
Organic–inorganic hybrid materials like metal–organic frameworks are a fascinating field of research. These metal–organic frameworks are built from metal ions and organic ligands which form bridges between the metal ions.1 These crystalline porous materials have unique properties of high surface areas with well defined cavities or channels2 that can be used in various potential applications including gas storage, separation, drug delivery, catalysis and others.3–6 MOFs have also been found to be potential candidates for other applications such as sensing,7 microelectronics,8 optics,9 pollutant sequestration,10 energy production,11 micro-motors,12 molecular rotors,13 bioreactors,14 diagnostics and controlled drug release.15
Nowadays, researchers are focusing on combining functional nano and microparticles with MOFs in order to fabricate nanocomposites which exhibit specific functional properties. Such nanoparticles include metal nanoparticles/nanorods,16–20 quantum dots (QDs),21 graphene,22,23 dense and porous silica nanospheres,24–26 and magnetic beads.23 In particular, the integration of magnetic materials into MOFs appears to be of considerable interest because the composites can be easily positioned by an external magnetic field.27 From a catalysis point of view, magnetic separation has many more advantages compared to filtration & centrifugation, liquid–liquid extraction, etc. D. P. Kim and co-workers examined the catalytic performance of Fe3O4@ZIF-8 using Knoevenagel condensation as a probe reaction.28
MIL-101(Cr) has mesosized cages of 2.9 and 3.4 nm with two microporous windows of 1.2 and 1.6 nm respectively,29,30 giving rise to a high surface area. The high surface area, mesoporosity and high hydrothermal stability collectively make this MOF a good candidate for a catalyst support.27
Many attempts have been made to synthesize nanocomposites of MIL-101 with magnetite nanoparticles. However, the preparation process involves the modification of magnetic components. Huo and Yan synthesized a magnetic framework composite by mixing MIL-101(Cr) with silica coated magnetite particles.31 Qiu and co-workers developed a liquid phase epitaxy (LPE) process for synthesizing Fe3O4@MIL-101(Fe) core–shell microspheres with different MOF shell thicknesses.32 J. Zhu and co-workers synthesized Fe3O4@MIL-100(Fe) magnetic microspheres and studied their catalytic activity for the Claisen–Schmidt condensation reaction.33
Benzaldehyde is a versatile compound used primarily as a precursor to other important organic compounds, varying from pharmaceuticals to fine chemicals. It is the expected product from the partial oxidation of benzyl alcohol. Kamonsatikul et al. demonstrated the oxidation of benzyl alcohol by Fe3O4 nanoparticles stabilized by ferrocene moieties.34 Hua Zhang and co-workers showed a higher catalytic activity and selectivity for the aqueous-phase aerobic oxidation of benzyl alcohol by a Fe3O4–Pt–rGO composite.35 Other catalysts reported for the oxidation of alcohol based on the principle of encapsulation include Pt@MOF-177,36 Au clusters on PCPs,37 Au@MOF,38 Pd@MIL-10139, etc. In this communication we report a facile route for synthesizing Fe3O4 nanoparticles on a MIL-101(Cr) support to get the Fe3O4@MIL-101(Cr) nanocomposite for the first time, and study its efficiency for solvent free oxidation of benzyl alcohol with tert-butyl hydroperoxide (TBHP) as the oxidant.
In a typical synthesis, 1 mmol of FeCl2·4H2O and 2 mmol of FeCl3 were added to an aqueous suspension (100 ml) containing 0.5 g of MIL-101(Cr). The mixture was then vigorously stirred and degassed with nitrogen for 1 h, followed by addition of an NH3 solution (15 ml) to form a black suspension. The resulting black solid was filtered off and repeatedly washed with double distilled water until the pH became neutral. After air drying the composite was designated as Fe3O4@MIL-101(Cr).
The formation of Fe3O4 nanoparticles in the nanocomposite was confirmed by low and wide angle powder XRD (ESI,† Fig. S1). Low-angle PXRD patterns of Fe3O4@MIL-101(Cr) together with the pattern of the parent MIL-101 are characteristic of MIL-101(Cr). The relative intensities of the diffraction peaks are decreased in the composite material, confirming encapsulation of Fe3O4 nanoparticles within the pores of MIL-101. The wide angle PXRD pattern of Fe3O4@MIL-101(Cr) can be readily indexed to crystalline Fe3O4 [JCPDS NO. 19-0629].
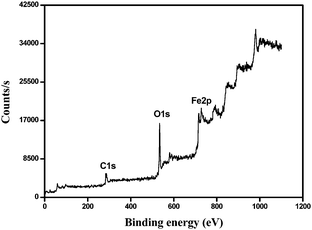
The FESEM images of MIL-101, Fe3O4 nanoparticles and Fe3O4@MIL-101(Cr) nanocomposites are shown in Fig. 1(a)–(c). It can be seen that uniform spherical Fe3O4 nanoparticles are incorporated onto the MIL-101 surface without fragmentation of the characteristic cubic symmetry of MIL-101(Cr). The integrity of the cubic symmetry of MIL-101(Cr) after generation of magnetite nanoparticles on its surface is also supported by the TEM images (Fig. S8, ESI†). The particle size distribution of the Fe3O4 nanoparticles supported on MIL-101 is shown in Fig. S9, ESI.† The elemental mapping (Fig. 1(d)) indicates the presence of Fe and Cr ions in the composites. The binding energies of the different elements of Fe3O4@MIL-101 are shown in the XPS spectrum (Fig. 2). The spectrum illustrates the presence of Fe3O4 nanoparticles and the binding energies of Fe2p3/2 and Fe2p1/2 are 715.80 eV and 728.85 eV respectively. The spectrum also shows peaks corresponding to the different elements present in the composite.
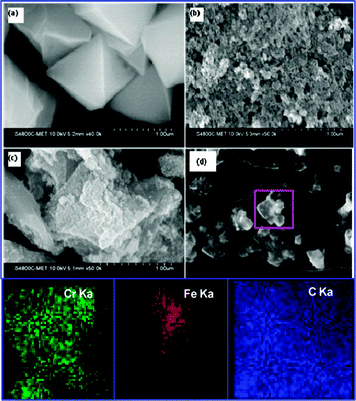 | ||
| Fig. 1 FESEM images of (a) MIL-101(Cr), (b) Fe3O4 nanoparticles, (c) Fe3O4@MIL-101(Cr) and (d) EDS mapping images of Fe3O4@MIL-101(Cr). | ||
Fourier transform infrared (FT-IR) spectra (ESI,† Fig. S4) show characteristic vibration bands at around 1400 and 1510 cm−1 for the framework (O–C–O)– groups, confirming the presence of the dicarboxylate moieties within MIL-101. The bands at around 3432 and 1618 cm−1 are owing to the water molecules within the pores of MIL-101. The characteristic broad band at 580 cm−1 for the nanocomposite confirms the incorporation of Fe–O groups on MIL-101(Cr).
The thermogravimetric analysis (TGA) curve (ESI,† Fig. S2) of MIL-101(Cr) exhibits two main weight loss steps: the first in the temperature range of 25–300 °C is associated with loss of the guest water molecules and the second between 300 °C–490 °C is related to the elimination of OH/F groups and framework decomposition. A similar weight loss pattern is also obtained for the composite material but with a strong decrease in the weight loss percentage, confirming the presence of some Fe3O4 particles within the pores of MIL-101(Cr). However, the composite material shows a greater weight loss at around 100 °C compared to the parent material due to more adsorption of water molecules on its surface.
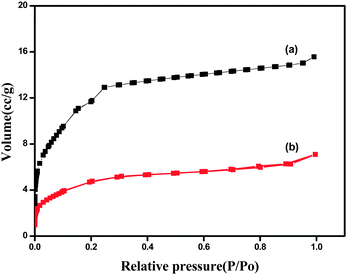
The N2 sorption isotherm shows two distinctive secondary uptakes at P/Po of around 0.1 and 0.2, which were caused by micropore window effects.40 The permanent porosity of MIL-101(Cr) was confirmed by the N2 adsorption isotherm (Fig. 3) exhibiting strong uptake at low relative pressures, due mostly to the microporous nature of the material.40 MIL-101(Cr) was found to possess a BET (Brunauer–Emmett–Teller) surface area of 2787 m2 g−1 and a pore volume of 1.58 cm−3 g−1 with an average pore diameter of 2.28 nm, showing resemblance to the nanosized MIL-101(Cr) prepared in the presence of HF.41
Fe3O4@MIL-101(Cr) has a lower BET surface area and pore volume than the parent material, which is anticipated from filling the pores of the parent material with Fe3O4 particles. Nevertheless, a significant BET surface area of 1439 m2 g−1 and pore volume of 0.97 cm3 g−1 were conserved.
A comparative study of the magnetic properties of the nanocomposite and Fe3O4 nanoparticles has been displayed (ESI,† Fig. S3). At room temperature no typical hysteresis loop is observed, indicating the superparamagnetic behaviour of the nanocomposite owing to aggregation of the small sized spherical magnetic particles.42 The saturation magnetization value of the nanocomposite is quite small as compared to the Fe3O4 nanoparticles which is consistent with the small particle size effect43 and the presence of the nonmagnetic MIL-101(Cr) support in the nanocomposite. This superparamagnetic behaviour of the nanocomposite permits the particles to aggregate rapidly in the presence of an external magnetic field, however the particles disperse easily as soon as the external field is removed.
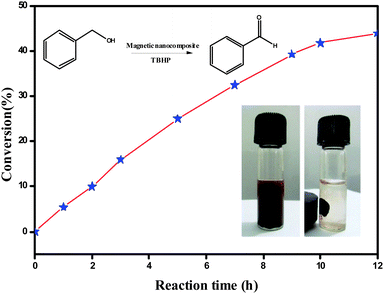
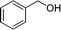
The catalytic performance of the Fe3O4@MIL-101(Cr) magnetic nanocomposite was evaluated through the solvent free oxidation of benzyl alcohol using tert-butyl hydroperoxide (TBHP) as the oxidant. The reaction was carried out by heating a mixture of benzyl alcohol (1 mmol) and TBHP (4 mmol) at 70 °C for 12 h under solvent free conditions in the presence of Fe3O4@MIL-101 (40 mg), which led to the formation of benzaldehyde with a 44% conversion and a 98% selectivity (Fig. 4). Three reaction cycles were tested in order to assess the stability and heterogeneous nature of the catalyst, and no deactivation of the catalyst was observed (ESI,† Fig. S6). The PXRD patterns of the recovered Fe3O4@MIL-101(Cr) (ESI,† Fig. S5) are identical to those of the freshly prepared nanocomposite. In each cycle, the catalyst was separated magnetically from the reaction vial, washed with ethanol and dried in an oven at 50 °C.
For comparison, we also carried out the oxidation reaction with pristine MIL-101 as well as with neat Fe3O4 nanoparticles under the same reaction conditions. In the case of pristine MIL-101 we didn't find any oxidation products while neat Fe3O4 showed some activity but the activity was much lower than that of Fe3O4@MIL-101.
This observation indicates that MIL-101 plays a major role as a support in this catalytic process by enhancing the catalytic activity of Fe3O4 nanoparticles. The high surface area of MIL-101 provides better dispersion for the Fe3O4 nanoparticles and hence we obtain good catalytic activity for the magnetic nanocomposite.
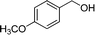
The optimized reaction conditions were employed for other benzyl alcohols containing different substituents, such as –Cl, –OMe, –Me, etc. The results are summarized in Table 1. For substituted benzyl alcohols we obtained comparatively better product conversions with high selectivities. We have also compared the catalytic activity of our material with those of some reference materials (ESI,† Table S1) in order to evaluate the efficiency of our catalyst.
| Entry | Substrates | Products | Conv. (%) | Selec. (%) |
|---|---|---|---|---|
| a Reaction conditions: benzyl alcohols (1 mmol), TBHP (4 mmol), Fe3O4@MIL-101(Cr) (40 mg), 70 °C, 12 h, solvent free, GC conversion. b Oxidation by MIL-101(Cr). c Oxidation by Fe3O4 nanoparticles. | ||||
| 1b |
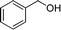
|
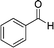
|
<2 | 85 |
| 2c |
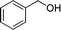
|
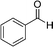
|
10 | 88 |
| 3 |
|
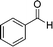
|
44 | 98 |
| 4 |
|
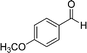
|
63.7 | 98.4 |
| 5 |
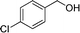
|
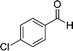
|
79.8 | 98.1 |
| 6 |
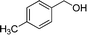
|
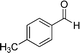
|
54 | 96.2 |
In summary, we have successfully synthesized magnetite nanoparticles on MIL-101(Cr). This nanocomposite acts as an efficient catalyst for solvent free oxidation of benzyl alcohol and substituted benzyl alcohols. The MIL-101(Cr) support plays a vital role in this catalytic conversion of benzyl alcohol to benzaldehyde by enhancing the catalytic activity of Fe3O4 nanoparticles. Moreover, the composite can easily be recycled from the reaction system by magnetic separation and no deactivation was observed after three cycles of reaction. The overall process is simple and rapid can be further extended to other MOFs which are chemically potent and thermally robust in nature.
Acknowledgements
The authors are grateful to the Director of CSIR-North East Institute of Science and Technology, Jorhat, Assam, India for his kind permission to publish the work. We are thankful to Dr Sasanka Deka and Kalyan Deori, Delhi University for field magnetization data and Dr Sudhir S. Arbuj, CMET, Pune for FESEM analysis. Thanks are also extended to Dr P. Sengupta, Head of Materials Science Division and Dr D. K. Dutta for support, and non network project MLP-6000(WP-1) and DST-RFBR joint project GPP-0287 for financial assistance. M. S. acknowledges DST New Delhi for an INSPIRE fellowship.Notes and references
- L. H. Wee, F. Bonino, C. Lamberti, S. Bordigab and J. A. Martensa, Green Chem., 2014, 16, 1351 RSC.
- M. S. E-Shall, V. Abdelsayed, A. E. R. S. Khder, H. M. A. Hassan, H. M. El-Kaderi and T. E. Reich, J. Mater. Chem., 2009, 19, 7625 RSC.
- Z. Wang, G. Chen and K. Ding, Chem. Rev., 2009, 109, 322 CrossRef CAS PubMed.
- J.-R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS PubMed.
- A. Corma, H. García and F. X. L. i Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed.
- A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. E. Gabaly, H. P. Yoon, F. Léonard and M. D. Allendorf, Science, 2014, 343, 66 CrossRef CAS PubMed.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed.
- C. M. Doherty, E. Knystautas, D. Buso, L. Villanova, K. Konstas, A. J. Hill, M. Takahashi and P. Falcaro, J. Mater. Chem., 2012, 22, 11470 RSC.
- S. K. Henninger, F. Jeremias, H. Kummer and C. Janiak, Eur. J. Inorg. Chem., 2012, 2625 CrossRef CAS.
- Y. Ikezoe, G. Washino, T. Uemura, S. Kitagawa and H. Matsui, Nat. Mater., 2012, 11, 1081 CAS.
- A. Comotti, S. Bracco, T. Ben, S. Qiu and P. Sozzani, Angew. Chem., Int. Ed., 2014, 53, 6655 CrossRef PubMed.
- C. M. Doherty, G. Grenci, R. Riccoò, J. I. Mardel, J. Reboul, S. Furukawa, S. Kitagawa, A. J. Hill and P. Falcaro, Adv. Mater., 2013, 25, 4701 CrossRef CAS PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232 CrossRef CAS PubMed.
- K. Sugikawa, S. Nagata, Y. Furukawa, K. Kokado and K. Sada, Chem. Mater., 2013, 25, 2565 CrossRef CAS.
- L. He, Y. Liu, J. Liu, Y. Xiong, J. Zheng, Y. Liu and Z. Tang, Angew. Chem., Int. Ed., 2013, 52, 3741 CrossRef CAS PubMed.
- Q.-L. Zhu, J. Li and Q. Xu, J. Am. Chem. Soc., 2013, 135, 10210 CrossRef CAS PubMed.
- H. R. Moon, D.-W. Lim and M. P. Suh, Chem. Soc. Rev., 2013, 42, 1807 RSC.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Nat. Chem., 2012, 4, 310 CrossRef CAS PubMed.
- D. Buso, J. Jasieniak, M. D. H. Lay, P. Schiavuta, P. Scopece, J. Laird, H. Amenitsch, A. J. Hill and P. Falcaro, Small, 2012, 8, 80 CrossRef CAS PubMed.
- C. Petit and T. J. Bandosz, Adv. Mater., 2009, 21, 4753 CAS.
- M. Jahan, Z. Liu and K. P. Loh, Adv. Funct. Mater., 2013, 23, 5363 CrossRef CAS.
- D. Buso, K. M. Nairn, M. Gimona, A. J. Hill and P. Falcaro, Chem. Mater., 2011, 23, 929 CrossRef CAS.
- A. Ahmed, M. Forster, R. Clowes, D. Bradshaw, P. Myers and H. F. Zhang, J. Mater. Chem. A, 2013, 1, 3276 CAS.
- S. Aguado, J. Canivet and D. Farrusseng, J. Mater. Chem., 2011, 21, 7582 RSC.
- R. Ricco, L. Malfatti, M. Takahashi, A. J. Hill and P. Falcaro, J. Mater. Chem. A, 2013, 1, 13033 CAS.
- M. Faustini, J. Kim, G.-Y. Jeong, J. Y. Kim, H. R. Moon, W.-S. Ahn and D.-P. Kim, J. Am. Chem. Soc., 2013, 135, 14619 CrossRef CAS PubMed.
- G. Ferey, C. M. Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040 CrossRef CAS PubMed.
- Y. K. Hwang, D. Y. Hong, J. S. Chang, S. H. Jhung, Y. K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Ferey, Angew. Chem., Int. Ed., 2008, 47, 4144 CrossRef CAS PubMed.
- S.-H. Huo and X.-P. Yan, Analyst, 2012, 137, 3445 RSC.
- F. Ke, L.-G. Qiu, Y.-P. Yuan, X. Jiang and J.-F. Zhu, J. Mater. Chem., 2012, 22, 9497 RSC.
- F. Ke, L.-G. Qiu and J. Zhu, Nanoscale, 2014, 6, 1596 RSC.
- C. Kamonsatikul, T. Khamnaen, P. Phiriyawirut, S. Charoenchaidet and E. Somsook, Catal. Commun., 2012, 26, 1 CrossRef CAS PubMed.
- S. Wu, Q. He, C. Zhou, X. Qi, X. Huang, Z. Yin, Y. Yang and H. Zhang, Nanoscale, 2012, 4, 2478 RSC.
- S. Proch, J. Herrmannsdörfer, R. Kempe, C. Kern, A. Jess, L. Seyfarth and J. Senker, Chem. – Eur. J., 2008, 14, 8204 CrossRef CAS PubMed.
- T. Ishida, M. Nagaoka, T. Akita and M. Haruta, Chem. – Eur. J., 2008, 14, 8456 CrossRef CAS PubMed.
- H. Liu, Y. Liu, Y. Li, Z. Tang and H. Jiang, J. Phys. Chem. C, 2010, 114, 13362 CAS.
- G. Chen, S. Wu, H. Liu, H. Jiang and Y. Li, Green Chem., 2013, 15, 230–235 RSC.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- C.-Y. Sun, S.-X. Liu, D.-D. Liang, K.-Z. Shao, Y.-H. Ren and Z.-M. Su, J. Am. Chem. Soc., 2009, 131, 1883 CrossRef CAS PubMed.
- (a) X.-H. Li, D.-H. Zhang and J.-S. Chen, J. Am. Chem. Soc., 2006, 128, 8382 CrossRef CAS PubMed; (b) H. Deng, X. Li, Q. Peng, X. Wang, J. Chen and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 2782 CrossRef CAS PubMed.
- (a) D. E. Zhang, X. J. Zhang, X. M. Ni, J. M. Song and H. G. Zheng, Cryst. Growth Des., 2007, 7, 2117 CrossRef CAS; (b) B. H. Sohn, R. E. Cohen and G. C. Papaefthyrniou, J. Magn. Magn. Mater., 1998, 182, 216 CrossRef CAS; (c) S. Santra, R. Tapec, N. Theodoropoulou, J. Dobson, A. Hebard and W. Tan, Langmuir, 2001, 17, 2900 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, synthetic procedures, PXRD, TGA, field magnetization, FT-IR, TEM images and XPS spectra. See DOI: 10.1039/c4nj01312c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |