Facile synthesis of mesoporous cobalt oxide rugby balls for electrochemical energy storage†
Yuting
Hao
,
Huanwen
Wang
,
Zhonghua
Hu
,
Lihua
Gan
and
Zijie
Xu
*
Department of Chemistry, Tongji University, Shanghai 200092, China. E-mail: xzj@tongji.edu.cn; Fax: +86 02165982287; Tel: +86 02165982654x8430
First published on 17th September 2014
Abstract
Mesoporous Co3O4 rugby balls were firstly obtained by utilizing a facile two-step method. This unique morphology together with the mesoporous feature manifests excellent capacitive performance an energy density (14.3 W h kg−1), a power density (7503 W kg−1) and no decay after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles for constructing the Co3O4 rugby balls//graphene hydrogels asymmetric supercapacitor.
000 cycles for constructing the Co3O4 rugby balls//graphene hydrogels asymmetric supercapacitor.
As a class of energy storage device with properties intermediate to those of batteries and electrostatic capacitors, supercapacitors display the desirable properties of high power density (ten times more than batteries), fast rates of charge–discharge (with seconds), excellent cycling stability, small size, low mass and low maintenance cost.1–3 However, the energy storage density of existing supercapacitors is limited, generally an order of magnitude lower than that of batteries. Currently, improving the energy density while maintaining the high power density and cycling stability for supercapacitor devices remains a primary research focus in the field.4,5
Pseudocapacitive transition-metal oxides such as RuO2,6 NiO,7 Co3O4,8 Bi2O39 and MnO2,10 have been studied extensively as active electrode materials for supercapacitors owing to their high energy density and large charge transfer-reaction pseudocapacitance which is based on fast and reversible redox reactions at the electrode surface, resulting in much higher specific capacitance exceeding that of carbon-based materials. Among them, Co3O4 is considered to be the most attractive oxide material due to its abundance, low cost, higher surface area, good redox property, controllable size and shape, and structural identities.11–13 Recent studies have shown supercapacitor applications of different Co3O4 nanostructures, including hollow spheres (346 F g−1),14 porous films (454 F g−1 at 2 A g−1),15 nanotubes (574 F g−1 at 0.1 A g−1),16 nanowires (1160 F g−1 at 2 A g−1),17 rectangular 2D flakes (548 F g−1 at 8 A g−1),18 hollow boxes (278 F g−1 at 0.5 A g−1),19 aerogels (623 F g−1),20 nanocone arrays (562 F g−1 at 2 A g−1),21 interconnected nanoflake films.22 As the capacitive phenomenon is directly associated with surface properties, any change in the surface morphology of the sample greatly influences its electrochemical performance. Toward theoretical Faradaic capacitance, seeking for a highly novel microstructure for Co3O4 has still been expected.
In this work, mesoporous Co3O4 rugby balls have been successfully and firstly prepared via a facile two-step method using Polyvinylpyrrolidone (PVP) as the structure-directing reagent. When used as supercapacitor electrodes, such particular morphology together with the mesoporous microstructure gives excellent capacitive performance during the charge–discharge and thus promising application as supercapacitors. On the basis of the as-prepared Co3O4 rugby balls and 3D conductive graphene hydrogels, an asymmetric supercapacitor is constructed and high energy density and power density as well as long cycling performance have been demonstrated.
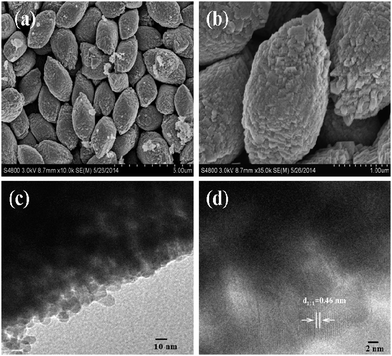
The surface morphology of as-synthesized Co3O4 is characterized by FESEM. Fig. 1a and b shows low- and high-magnification FESEM images. It is observed that the Co3O4 sample exhibits a uniform rugby ball morphology. The size of single Co3O4 rugby ball is 1 μm and 2 μm in the transverse and longitudinal direction, respectively. From TEM images, it can be see that each rugby ball consists of numerous interconnected nanoparticles forming a mesoporous structure, which is generated during the thermal treatment (Fig. 1c). The high-resolution TEM (HRTEM) image shown in Fig. 1d further reveals these Co3O4 nanoparticles with a size of ca. 10 nm and the existence of interparticle mesopores with a size ranging from 2 to 5 nm. The lattice fringes show an interplanar spacing of ca. 0.46 nm (Fig. 1d), corresponding to the (111) plane of the cubic Co3O4.
From the XRD pattern (Fig. 2a), it can be seen that the sample shows typical diffraction peaks of the Co3O4 phase (JCPDS, No. 43-1003). In Fig. 2b, five distinguishable Raman bands are located at approximately 194, 473, 518, 615, and 681 cm−1, which correspond to the F2g, Eg, F2g, F2g and A1g modes, respectively, of the crystalline Co3O4 phase in agreement with the literature values.23 From the N2 adsorption–desorption isotherm of the Co3O4 rugby balls (Fig. 2c), it can be seen that a distinct hysteresis loop can be found with typical IV sorption behavior, indicating the existence of a typical mesoporous microstructure. The pore-size-distribution data (Fig. 2d) show that the size of majority of the pores is 8.9 nm. The mesoporosity is assigned to the thermal decomposition of the starting material, which could result in weight loss and volume shrinking. The mesoporous structure gives rise to a relatively high Brunauer–Emmett–Teller (BET) specific surface area of 61 m2 g−1.
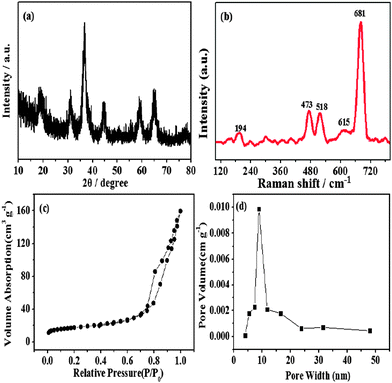 | ||
| Fig. 2 (a) XRD patterns, (b) Raman spectrum, (c) nitrogen adsorption and desorption isotherms and (d) BJH pore size distribution for mesoporous cobalt oxide rugby balls. | ||
Such unique Co3O4 rugby ball morphology coupled with the mesoporous structure and high surface area are beneficial to ion transport in the electrode/electrolyte. The electrochemical performance is obtained by cyclic voltammetry (CV) and galvanostatic charge–discharge measurements. From CV curves (Fig. 3a), there are two redox peaks due to the following faradaic reactions:24–26
| Co3O4 + OH− ↔ 3CoOOH + e− | (1) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (2) |
Fig. 3b presents galvanostatic charge–discharge curves of the mesoporous Co3O4 rugby balls at different current densities. The specific capacitance is shown as a function of the current density in Fig. 3c. At 1 A g−1, Co3O4 rugby balls have a capacitance value of 480 F g−1. Even at a high specific current of 10 A g−1, there is still 64% capacitance retention, which is indicative of the excellent rate property of the Co3O4 rugby ball electrode. The excellent cycling stability is demonstrated in Fig. 3d. At a cycle number of 150, the capacitance value increases 18% because of sample activation. After a 3000 cycle test, the capacity retains 84% of the initial value.
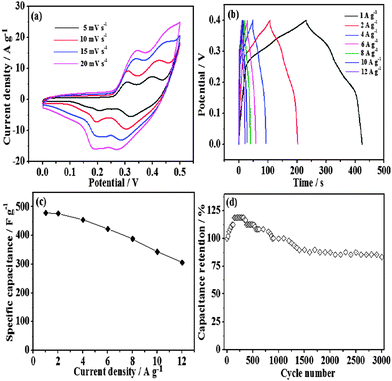 | ||
| Fig. 3 (a) CV curves, (b) charge–discharge curves, (c) capacitances versus current densities and (d) variation of capacitance with cycle number at 4 A g−1 of mesoporous cobalt oxide rugby balls. | ||
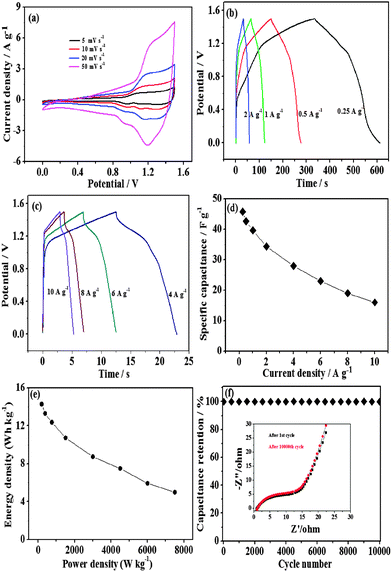
To further investigate the practical application of the sample in electrochemical energy storage, we construct a novel asymmetric supercapacitor in aqueous KOH solution. The positive electrode is the Co3O4 rugby balls. In addition, considering the multiple advantages of graphene hydrogels in comparison with other carbon materials, including high surface-area-to-volume ratios, enhanced transport property and mechanical flexibility, it is worthwhile to select graphene hydrogels as the negative electrode for the asymmetric capacitor. From the FESEM images, it can be seen that graphene hydrogels have a macroporous morphology with the framework network (Fig. 4a). In the potential range from −0.9 to 0 V at a scan rate of 5 to 50 mV s−1, CV curves of graphene hydrogels exhibit a typical rectangular shape, which shows good charge propagation at the electrode surface (Fig. 4b). According to the charge–discharge curves (Fig. 4c), the specific capacitance of graphene hydrogels is calculated to be 200 F g−1 at a current density of 1 A g−1. More importantly, graphene hydrogels still have a capacitance of 138 F g−1 at 20 A g−1 with 56% retention relative to 1 A g−1. These capacitance performances are superior to previous activated carbon (AC).27
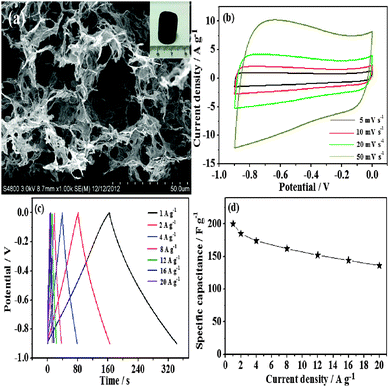 | ||
| Fig. 4 (a) FESEM images, (inset) the photograph, (b) CV curves, (c) charge–discharge curves, and (d) capacitances versus current densities of graphene hydrogels. | ||
On the basis of the Co3O4 rugby balls (positive) and graphene hydrogels (negative), the asymmetric supercapacitor is constructed in 1 M KOH solution (Fig. 5). The asymmetric supercapacitors usually consist of a Faradaic electrode (as the energy source) and a capacitor-type electrode (as the power source), which can make full use of the different potential windows of the two electrodes to provide a maximum operation voltage in the cell system, and accordingly result in a greatly improved energy density. For asymmetric supercapacitors, it is well-known that the charge balance between the two electrodes will follow the relationship q+ = q−, where the charge stored by each electrode usually depends on the specific capacitance (C), the potential range for the charge–discharge process (ΔE), and the mass of the electrode (m) following equation: q = C × ΔE × m and in order to obtain q+ = q−, the mass balancing will be expressed as follows:
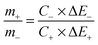 | (3) |
Based on the above analysis of the specific capacitance values and potential ranges found for the Co3O4 rugby balls and porous graphene hydrogels, the optimal mass ratio between the two electrodes should be m(Co3O4)/m(graphene) = 1 in the asymmetric supercapacitor cell.
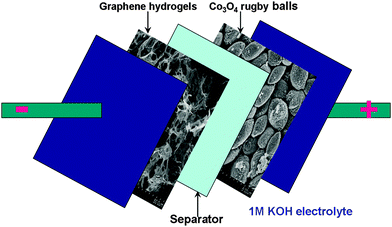 | ||
| Fig. 5 Schematic of the assembled structure of the Co3O4 rugby balls//graphene hydrogels asymmetric supercapacitor. | ||
The electrochemical performance of the Co3O4 rugby balls//graphene hydrogels asymmetric supercapacitor is shown in Fig. 6. The operating cell voltage can be extended to approximately 1.5 V. Obvious redox peaks are observed (Fig. 6a), indicating the Faradaic pseudocapacitive nature of the Co3O4 rugby balls//graphene hydrogels capacitor arising from the Co3O4 rugby ball electrode. All the discharge curves are nearly symmetric to their corresponding charging counterparts (Fig. 6b and c), suggesting an excellent electrochemical reversibility. The relationship between specific capacitance and current density is illustrated in Fig. 6d. The energy density, E = [C(ΔV)2]/2, and power density, P = E/Δt, are two important parameters that characterize the supercapacitor, where C, ΔV and Δt are specific capacitance, potential window and discharge time, respectively.28,29 The calculation is base on the total mass of the positive (the Co3O4 rugby balls) and negative (graphene hydrogels) electrodes. Fig. 6e presents a Ragone plot, which relates the energy density to the power density of the asymmetric capacitor. The energy density decreases from 14.3 to 5 W h kg−1 as the power density increases from 185 to 7503 W kg−1. Noticeably, the energy density of 14.3 W h kg−1 is much higher than that of symmetrical supercapacitors based on hydrazine reduced graphene hydrogels (5.7 W h kg−1).30 Furthermore, after a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycle test at 4 A g−1, the Co3O4 rugby balls//graphene hydrogels supercapacitor shows no obvious decay (Fig. 5f). This cycling performance is superior to most of the previously reported samples including Ni(OH)2//AC (82% retention after 1000 cycles),31 graphene/MnO2//graphene (79% retention after 1000 cycles),32 NiCo2O4–rGO//AC (83% after 2500 cycles).33 EIS measurements after the 1st and 10
000 cycle test at 4 A g−1, the Co3O4 rugby balls//graphene hydrogels supercapacitor shows no obvious decay (Fig. 5f). This cycling performance is superior to most of the previously reported samples including Ni(OH)2//AC (82% retention after 1000 cycles),31 graphene/MnO2//graphene (79% retention after 1000 cycles),32 NiCo2O4–rGO//AC (83% after 2500 cycles).33 EIS measurements after the 1st and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000th cycle are performed at 1.2 V. As shown in the inset of Fig. 6f, the impedance plots of the asymmetric supercapacitor show no obvious difference after the 10
000th cycle are performed at 1.2 V. As shown in the inset of Fig. 6f, the impedance plots of the asymmetric supercapacitor show no obvious difference after the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000th cycle. This further indicates that repetitive cycling does not induce noticeable degradation of the microstructure. These superior electrochemical performances may be due to the synergistic effect of the Co3O4 rugby balls and graphene hydrogels.
000th cycle. This further indicates that repetitive cycling does not induce noticeable degradation of the microstructure. These superior electrochemical performances may be due to the synergistic effect of the Co3O4 rugby balls and graphene hydrogels.
In summary, we firstly synthesized mesoporous Co3O4 rugby balls using a simple solvothermal/calcination process. Such rugby balls possess excellent electrochemical performance and are suitable as advanced electrode materials for pseudosupercapacitors. When the mesoporous Co3O4 rugby balls and 3D graphene hydrogels are formed to be an asymmetric supercapacitor, high energy density and power density, as well as a super-long cycle life have been achieved. These findings promote new opportunities for mesoporous Co3O4 rugby balls as high-performance supercapacitors and other energy-storage devices.
Acknowledgements
The authors would like to thank the Chemistry Experimental Centre at the Tongji University for providing characterization equipment and assistance.Notes and references
- J. R. Miller, R. A. Outlaw and B. C. Holloway, Science, 2010, 329, 1637 CrossRef CAS PubMed.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294 CAS.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472 CrossRef CAS PubMed.
- W. Chen, R. B. Rakhi, L. Hu, X. Xie, Y. Cui and H. N. Alshareef, Nano Lett., 2011, 11, 5165 CrossRef CAS PubMed.
- Y. Lu, X. Wang, Y. Mai, J. Xiang, H. Zhang, L. Li, C. Gu, J. Tu and S. X. Mao, J. Phys. Chem. C, 2012, 116, 22217 CAS.
- C. C. Hu, K. H. Chang, M. C. Lin and Y. T. Wu, Nano Lett., 2006, 6, 2690 CrossRef CAS PubMed.
- H. Wang, Y. Wang and X. Wang, Electrochem. Commun., 2012, 18, 92 CrossRef CAS PubMed.
- R. Tummala, R. K. Guduru and P. S. Mohanty, J. Power Sources, 2012, 209, 44 CrossRef CAS PubMed.
- H. W. Wang, Z. A. Hu, Y. Q. Chang, Y. L. Chen, Z. Q. Lei, Z. Y. Zhang and Y. Y. Yang, Electrochim. Acta, 2010, 55, 8974 CrossRef CAS PubMed.
- H. Xia, M. O. Lai and L. Lu, J. Power Sources, 2011, 196, 2398 CrossRef CAS PubMed.
- M. B. Zheng, J. Cao, S. T. Liao, J. S. Liu, H. Q. Chen, Y. Zhao, W. J. Dai, G. B. Ji, J. M. Cao and J. Tao, J. Phys. Chem. C, 2009, 113, 3887 CAS.
- L. R. Hou, C. Z. Yuan, L. Yang, L. F. Shen, F. Zhang and X. G. Zhang, RSC Adv., 2011, 1, 1521 RSC.
- Y. Wang, Z. Y. Zhong, Y. Chen, C. T. Ng and J. Y. Lin, Nano Res., 2011, 4, 695 CrossRef CAS PubMed.
- D. Zhang and W. Zou, Curr. Appl. Phys., 2013, 13, 1796 CrossRef PubMed.
- B. R. Duan and Q. Cao, Electrochim. Acta, 2012, 64, 154 CrossRef CAS PubMed.
- J. Xu, L. Gao, J. Y. Cao, W. C. Wang and Z. D. Chen, Electrochim. Acta, 2010, 56, 732 CrossRef CAS PubMed.
- F. Zhang, C. Yuan, X. Lu, L. Zhang, Q. Chea and X. Zhang, J. Power Sources, 2012, 203, 250 CrossRef CAS PubMed.
- S. K. Meher and G. R. Rao, J. Phys. Chem. C, 2011, 115, 15646 CAS.
- W. Du, R. Liu, Y. Jiang, Q. Lu, Y. Fan and F. Gao, J. Power Sources, 2013, 227, 101 CrossRef CAS PubMed.
- T. Y. Wei, C. H. Chen, K. H. Chang, S. Y. Lu and C. C. Hu, Chem. Mater., 2009, 21, 3228 CrossRef CAS.
- F. Cao, G. X. Pan, P. S. Tang and H. F. Chen, J. Power Sources, 2012, 216, 395 CrossRef CAS PubMed.
- Y. F. Yuan, X. H. Xi, J. B. Wu, X. H. Huang, Y. B. Pei, J. L. Yang and S. Y. Guo, Electrochem. Commun., 2011, 13, 1123 CrossRef CAS PubMed.
- S. L. Xiong, C. Z Yuan, X. G. Zhang, B. J. Xi and Y. T. Qian, Chem. – Eur. J., 2009, 15, 5320 CrossRef CAS PubMed.
- Y. Wang, H. Wang and X. Wang, Electrochim. Acta, 2013, 92, 298 CrossRef CAS PubMed.
- X. Xia, J. Tu, Y. Zhang, X. Wang, C. Gu, X. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531 CrossRef CAS PubMed.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- X. Xia, D. Chao, Z. Fan, C. Guan, X. Cao, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 1651 CrossRef CAS PubMed.
- H. Wang, H. Yi, X. Chen and X. Wang, J. Mater. Chem. A, 2014, 2, 3223 CAS.
- L. Zhang and G. Shi, J. Phys. Chem. C, 2011, 115, 17206 CAS.
- J. W. Lang, L. B. Kong, M. Liu, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2010, 14, 1533 CrossRef CAS.
- Z. S. Wu, W. Ren, D. W. Wang, F. Li, B. Liu and H. M. Cheng, ACS Nano, 2010, 4, 5835 CrossRef CAS PubMed.
- X. Wang, W. S. Liu, X. H. Lu and P. S. Lee, J. Mater. Chem., 2012, 22, 23114 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c4nj01116c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |