Bridging homogeneous and heterogeneous catalysis with CAN·SiO2 as a solid catalyst for four-component reactions for the synthesis of tetrasubstituted pyrroles†
Amol B.
Atar
,
Jong Su
Kim
,
Kwon Taek
Lim
and
Yeon Tae
Jeong
*
Department of Image Science and Engineering, Pukyong National University, Busan 608-737, Republic of Korea. E-mail: ytjeong@pknu.ac.kr; Fax: +82 51 629 6408
First published on 31st October 2014
Abstract
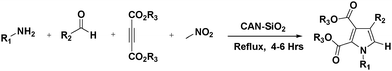
An efficient and expeditious method has been developed for the synthesis of tetrasubstituted pyrroles via a four component, one-pot cyclocondensation reaction of amines, aldehydes, dialkyl acetylenedicarboxylates and nitroalkanes using silica supported ceric ammonium nitrate as a heterogeneous catalyst for the first time. Reusable silica supported ceric ammonium nitrate was found to be a highly efficient and renewable heterogeneous catalyst for the rapid and convenient synthesis of tetrasubstituted pyrrole derivatives. The advantageous features of this novel methodology are high atom-economy, operational simplicity, shorter reaction times, convergence, and facile automation.
1. Introduction
Polysubstituted pyrroles represent some of the most important classes of heterocyclic molecules found in numerous natural and biologically active compounds.1 Polysubstituted pyrroles have received considerable attention in the field of synthetic organic chemistry due to their numerous applications in the pharmaceutical industry.2 Functionalized pyrroles have shown interesting biological properties such as antibacterial,3 antiinflammatory,4 antioxidant,5 antitumor, antifungal6 and immune suppressant activities.7 Highly functionalized pyrroles are subunits of heme, chlorophyll, bile pigments, vitamin B12 and pyrrole alkaloids isolated from the marine source.8 Furthermore, substituted pyrroles are widely used as synthetic building blocks, pharmacophores, and various kinds of functional materials.9 These compounds have many applications as chemosensors, for laser manufacture, and image diagnosis.10The importance of chemical and pharmacological properties of pyrrole derivatives and the development of synthetic methods, which enable a facile access to these heterocyclic compounds, are desirable. Recently, many efforts have been devoted to develop novel and highly efficient synthetic protocols for the synthesis of functionalized pyrroles such as multicomponent coupling,11 tandem reactions, transition-metal-catalyzed cyclization,12 and catalytic C–H bond functionalization strategies have been developed and drawn extensive and enduring attention.13 In the midst of them, multicomponent processes have received great attention from the chemical community because they address fundamental principles of synthetic efficiency and reaction processing.14 Multicomponent coupling reactions (MCRs) are known as powerful tools for the construction of novel and structurally complex molecules in a single pot ensuring high atom economy, good overall yields and high selectivity, lower costs, shorter reaction times, minimizing waste, labor, energy, and avoidance of expensive purification processes.15
Recently, a four-component reaction of amines, aldehydes, dialkyl acetylenedicarboxylates and nitroalkanes is reported to give diversity oriented pyrroles by using iodine as a catalyst.16 But unfortunately, they used homogenous catalysts and are not recyclable. The use of homogeneous catalysts has received little attention as alternatives in alleviating some of the limitations. Thus, the search for an inexpensive, readily available, and convenient catalyst is desirable. The use of heterogeneous catalysts17 has received considerable importance in organic synthesis because of their ease of handling, enhanced reaction rates, greater selectivity, simple work-up, and recoverability of catalysts. Among the various heterogeneous catalysts, particularly, silica gel-supported ceric ammonium nitrate18 has the advantages of low cost, ease of preparation and catalyst recycling. It is evident from the previous literature that silica gel supported ceric ammonium nitrate has invoked enormous interest as a green and a potential acid catalyst to construct carbon–carbon and carbon–heteroatom bonds in various organic transformations.19
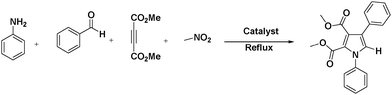
As part of our ongoing research program for the development of new environmentally benign methodology for the synthesis of a useful precursor in the field of biology, industry, and a key intermediate for the multistep synthesis,20–23 we decided to investigate the efficiency of a silica gel supported ceric ammonium nitrate catalyst for the synthesis of functionalized pyrroles. The reaction is easy to perform and allows synthesizing various multi functionalized derivatives. These reaction workup procedures are simple and we can isolate products with high purity and yields. So herein, we wish to report a tandem synthesis of tetrasubstituted pyrrole derivatives by using CAN·SiO2 as an expeditious reusable catalyst in an excellent yield (Scheme 1).
 | ||
| Scheme 1 Synthesis of fully substituted tetrasubstituted pyrrole functionalities catalyzed by CAN·SiO2. | ||
2. Results and discussion
To identify the suitable conditions for the four-component annulation process, a series of catalysts and solvents were screened (Table 1). Initially, in search of the best catalytic system for this one-pot synthesis, optimization of various reaction parameters like different metal Lewis acid catalysts, temperature, and solvents was carried out (Table 1) with the standard reaction of benzaldehyde, aniline, dimethyl acetylenedicarboxylates, and nitromethane. In order to establish the real effectiveness of the catalyst for the synthesis of tetra substituted pyrroles, a test reaction was performed without catalysts using the model reaction under reflux conditions. It was found that only a trace amount of product was obtained in the absence of a catalyst even after 24 h (Table 1, entry 1).| Entry | Catalyst (10 mol%) | Time (h) | Yieldb (%) |
|---|---|---|---|
| a All reactions were carried out using benzaldehyde (1 mmol), aniline (1 mmol), dimethyl acetylenedicarboxylates (1 mmol) and nitromethane (1 mL) under reflux conditions. b Yields obtained after column chromatography. | |||
| 1 | — | 24 | Traces |
| 2 | SiO2 | 12 | 24 |
| 3 | BF3·SiO2 | 10 | 48 |
| 4 | Cu(OTf)2·SiO2 | 12 | 51 |
| 5 | FeCl3·SiO2 | 8 | 75 |
| 6 | TiO2·SiO2 | 12 | 52 |
| 7 | ZnCl2·SiO2 | 8 | 60 |
| 8 | CAN | 4 | 91 |
| 9 | CAN·SiO2 | 4 | 93 |
| 10 | Zn(OTf)2·SiO2 | 9 | 65 |
| 11 | P2O5·SiO2 | 12 | 55 |
| 12 | FeCl3·SiO2 | 24 | 85 |
| 13 | CAN·SiO2 | 24 | 90 |
| 14 | CAN·SiO2 (5 mol%) | 4 | 85 |
| 15 | CAN·SiO2 (7 mol%) | 4 | 88 |
| 16 | CAN·SiO2 (15 mol%) | 6 | 90 |
| 17 | CAN·SiO2 (20 mol%) | 5 | 89 |
In search of an effective, eco-friendly, and efficient reusable catalytic system for this reaction, the same test reaction was performed with different metal Lewis acid catalysts such as SiO2, BF3·SiO2, Cu(OTf)2·SiO2, FeCl3·SiO2, TiO2·SiO2, ZnCl2·SiO2, CAN, CAN·SiO2, Zn(OTf)2·SiO2 and P2O5·SiO2 (Table 1). To study the role of SiO2, the reaction was tested with SiO2 (100 mg) only, and we observed a good yield as compared to the catalyst free reaction (Table 1, entry 2). Among all screened catalysts CAN and CAN·SiO2 gave the best results in view of yield and reaction times (Table 1, entries 8 and 9). In contrast, BF3·SiO2, Cu(OTf)2·SiO2, FeCl3·SiO2, TiO2·SiO2, ZnCl2·SiO2, Zn(OTf)2·SiO2 and P2O5·SiO2 did not afford the desired product in good yields (Table 1, entries 3–7, 10 and 11). The supported Lewis acid catalyst, FeCl3·SiO2, is effective, but it required more time for the completion of the reaction as compared with a CAN·SiO2 catalyst to slightly lower the yield (Table 1, entries 5, 9 and 12). The reaction was also studied in various solvents such as tetrahydrofuran, dichloromethane, dichloroethane, and toluene; however, the yield of the desired product was decreased. So, in this reaction nitromethane was used both as a solvent and as one of the reactants as well.
Once we found CAN·SiO2 as the best catalyst for this system, the optimization of catalyst loading was done (Table 1, entries 14–17). Consequently, the optimum 10 mol% loading of CAN·SiO2 provided the maximum yield in minimum time (Table 1, entry 9), but while loading a high amount of catalyst, the reaction proceeded quite slowly with a lower yield (Table 2, entries 16 and 17), on the other hand, a decrease in the yield was observed by lowering the amount of catalyst (Table 1, entries 14 and 15). So, 10 mol% of the catalyst were found to be the optimal quantity and sufficient to push the reaction forward.
| Entry (compound) | R1 | R2 | R3 | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a All reactions were carried out using amines (1 mmol), aldehydes (1 mmol), dialkyl acetylenedicarboxylates (1 mmol) and nitromethane (1 mL) under reflux conditions. b Isolated yield. | |||||
| 1 | Ph | Ph | Me | 4 | 93 |
| 2 | 2-CH3C6H4 | Ph | Et | 5 | 92 |
| 3 | 4-OCH3C6H4 | 2-CH3C6H4 | Et | 5 | 91 |
| 4 | 3-CH3C6H4 | Ph | Et | 4 | 93 |
| 5 | 4-CH3C6H4 | Ph | Et | 5 | 90 |
| 6 | 3-OCH3C6H4 | Ph | Et | 6 | 89 |
| 7 | 4-CH3C6H4 | 4-OHC6H4 | Me | 5 | 88 |
| 8 | 4-OCH3C6H4 | Ph | Et | 4 | 90 |
| 9 | PhCH2 | 4-OEtC6H4 | Me | 4 | 96 |
| 10 | 2-OCH3C6H4 | Ph | Et | 6 | 85 |
| 11 | 4-ClC6H4 | Ph | Et | 5 | 87 |
| 12 | 4-BrC6H4 | Ph | Et | 4 | 88 |
| 13 | 4-OCH3C6H4 | 4-FC6H4 | Me | 5 | 92 |
| 14 | 3,5-OCH3C6H3 | Ph | Et | 6 | 86 |
| 15 | 3,4-CH3C6H3 | Ph | Et | 5 | 90 |
| 16 | PhCH2 | 4-BrC6H4 | Me | 4 | 94 |
| 17 | PhCH2 | 3-FC6H4 | Me | 5 | 95 |
| 18 | 4-OCH3C6H4 | 3,4-FC6H3 | Me | 4 | 93 |
| 19 | 3,5-CH3C6H3 | Ph | Et | 5 | 89 |
| 20 | 2,5-CH3C6H3 | Ph | Et | 6 | 86 |
| 21 | 3,4-CH3C6H3 | Ph | Me | 4 | 92 |
| 22 | Ph | Thiophene | Me | 6 | 62 |
| 23 | PhCH2 | 4-CH3C6H4 | Me | 4 | 95 |
| 24 | 3,4-OCH3C6H3 | Ph | Et | 4 | 92 |
| 25 | PhCH2 | 3-FC6H4 | Et | 4 | 90 |
| 26 | 5-Amino indane | Ph | Me | 6 | 88 |
| 27 | 4-OCH3C6H4 | 2,3-FC6H3 | Me | 6 | 85 |
| 28 | 2,4,6-CH3C6H2 | Ph | Et | 6 | 82 |
| 29 | Ph | CH3CH2 | Me | 24 | Traces |
| 30 | H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2 CH–CH2 |
Ph | Et | 4 | 75 |
| 31 | PhCH2 | 4-BrC6H4 | Et | 5 | 89 |
| 32 | 5-Amino indane | Ph | Et | 4 | 84 |
To explore the scope of this novel methodology, reactions of several of amines, aldehydes, dialkyl acetylenedicarboxylates and nitromethane in the presence of 10 mol% of CAN·SiO2 were conducted. The results are summarized in Table 2. Thus, we selected the optimized reaction condition to examine the universality of this catalyst application with different electron rich and deficient substrates. It was gratifying to observe that most of the tested substrates exhibited satisfactory reactivity profiles, in all cases leading to a heterocyclization sequence that readily afforded the target structures (Table 2). But, with an aliphatic aldehyde only a trace amount of product was detected (Table 2, entry 29). On the other hand, in the case of amines, both aromatic and aliphatic substrates underwent the conversion smoothly.
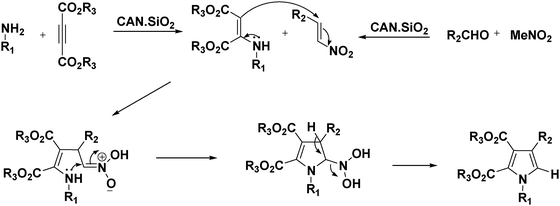
A possible mechanism of this one-pot reaction is expected on the basis of the reported literature.17 A possible mechanism for the tetrasubstituted pyrrole ring formation of four-component coupling reactions using CAN·SiO2 as a catalyst is outlined in Scheme 2.
The reusability of the CAN·SiO2 catalyst was also examined. The catalyst was reused four times and the results show that silica supported ceric ammonium nitrate can be reused as such without a significant loss in yield (Table 3). The recyclability of the catalyst was checked with the model reaction (Table 3, entries 1–4). Accordingly, after the first fresh run with a 93% yield, the catalyst was removed by filtration. The recovered catalyst was dried under vacuum at 120 °C for 12 h and tested up to three more reaction cycles. Recycling and reuse of the catalyst showed a minimal decrease in yields. The product was obtained in 93%, 93%, 91%, and 90% yields after successive cycles (Table 3, entries 1–4), thus proving the catalyst reusability. The catalyst showed excellent recyclability in all these reactions (Table 3), as the reaction times and yields remained almost the same without having a loss of catalytic activity.
The reported as well as synthesized novel compounds were further characterized by their spectral properties (1H, 13C NMR, and HRMS).
3. Conclusion
In summary, an efficient, expeditious, operationally simple, economical, and environmentally friendly method has been developed for the synthesis of tetra-substituted pyrroles via a four component; one-pot reaction of amines, aldehydes, dialkyl acetylenedicarboxylates, and nitromethane using silica supported ceric ammonium nitrate as a heterogeneous catalyst. The main advantage of this present methodology is the simple work-up, easy recovery of catalysts, no need for anhydrous conditions, and no base or any additional activator required. To the best of our knowledge this is the first report for the synthesis of tetra-substituted pyrroles by using silica supported ceric ammonium nitrate as a heterogeneous catalyst. Shorter reaction times, excellent yields, and more importantly, the recyclability without losing catalytic activity make this protocol good and attractive.4. Experimental
4.1 Material and methods
Chemicals were purchased from Aldrich and Alfa Aesar chemical companies and used as it is. The NMR spectra were recorded in CDCl3 on a Jeol JNMECP 400 NMR instrument using TMS as an internal standard. The HRMS was recorded on a Jeol JMS-700 mass spectrometer.4.2 General procedure for the synthesis of a silica supported ceric ammonium nitrate catalyst
A silica supported ceric ammonium nitrate catalyst was prepared by adopting the literature procedure.24 Neutral silica gel (9.01 g, Merck Kieselgel 60, particle size 0.063–0.200 mm, 70–230 mesh) was mixed with a solution of CAN (1.02 g) in water (2.0 mL). Evaporation of water under reduced pressure gave a dry yellowish powder, which contained 10% (by weight) of CAN. According to Hwu et al., this reagent was found active for at least six months by storing in a well-capped bottle.24References
- (a) Pyrroles, Part II, ed. R. A. Jones, Wiley, New York, 1992 Search PubMed; (b) Comprehensive Heterocyclic Chemistry II, ed. R. J. Sundberg, A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon Press, Oxford, UK, 1996, vol. 2, p. 119 Search PubMed; (c) A. H. Lipkus, Q. Yuan, K. A. Lucas, S. A. Funk, W. F. Bartelt, R. J. Schenck and A. J. Trippe, J. Org. Chem., 2008, 73, 4443 CrossRef CAS PubMed; (d) H. Fan, J. Peng, M. T. Hamann and J.-F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed; (e) C. T. Walsh, S. Garneau-Tsodikova and A. R. Howard-Jones, Nat. Prod. Rep., 2006, 23, 517 RSC; (f) I. S. Young, P. D. Thornton and A. Thompson, Nat. Prod. Rep., 2010, 27, 1801 RSC.
- E. Bellur, I. Freifeld and P. Langer, Tetrahedron Lett., 2005, 47, 2151–2154 CrossRef PubMed.
- G. Daidone, B. Maggio and D. Schillaci, Pharmazie, 1990, 45, 441–442 CAS.
- (a) A. Kimura, T. Kawara, A. Nakao, A. Ushiyama, S. Shimozato and T. K. Suzuki, PCT Int Appl. CODEN:PIXXD2, WO 2000001688 A1, 200001132000, 2000, p.173 Search PubMed; (b) D. G. Kaiser and E. M. Glenn, J. Pharm. Sci., 1972, 61, 1908–1911 CrossRef CAS.
- A. S. Demir, I. M. Akhmedov and O. Sesenoglu, Tetrahedron, 2002, 58, 9793–9799 CrossRef CAS.
- H. M. Meshram, B. R. V. Prasad and D. A. Kumar, Tetrahedron Lett., 2010, 51, 3477–3480 CrossRef CAS PubMed.
- F. A. Davis, K. Bowen, H. Xu, V. Velvadapu and C. Ballard, Tetrahedron, 2008, 64, 4174–4182 CrossRef CAS PubMed.
- M. Reisser and G. Maas, J. Org. Chem., 2004, 69, 4913–4924 CrossRef CAS PubMed.
- (a) B. A. Trofimov and N. A. Nedolya, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier, Oxford, UK, 2008, vol. 3, p. 45 Search PubMed; (b) X. L. Hou, Z. Yang and H. N. C. Wong, Prog. Heterocycl. Chem., 2003, 15, 167 CAS; (c) S. Yamaguchi and K. Tamao, J. Organomet. Chem., 2002, 653, 223 CrossRef CAS; (d) V. M. Domingo, C. Aleman, E. Brillas and L. Julia, J. Org. Chem., 2001, 66, 4058 CrossRef CAS; (e) P. A. Gale, Acc. Chem. Res., 2006, 39, 465 CrossRef CAS PubMed; (f) S. Michlik and R. Kempe, Nat. Chem., 2013, 5, 140 CrossRef CAS PubMed; (g) D. Srimani, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2013, 52, 4012 CrossRef CAS PubMed; (h) M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 6958 CrossRef CAS PubMed.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed.
- (a) X. Liu, L. Huang, F. Zheng and Z. Zhan, Adv. Synth. Catal., 2008, 350, 2778 CrossRef CAS; (b) D. J. Cyr and B. A. Arndtsen, J. Am. Chem. Soc., 2007, 129, 12366 CrossRef PubMed; (c) Y. Lu and B. A. Arndtsen, Angew. Chem., Int. Ed., 2008, 47, 5430 CrossRef CAS PubMed; (d) W. Liu, H. Jiang and L. Huang, Org. Lett., 2010, 12, 312 CrossRef CAS PubMed; (e) S. Maiti, S. Biswas and U. Jana, J. Org. Chem., 2010, 75, 1674 CrossRef CAS PubMed.
- (a) H. Y. Wang, D. S. Mueller, R. M. Sachwani, R. Kapadia, H. N. Londino and L. L. Anderson, J. Org. Chem., 2011, 76, 3203 CrossRef CAS PubMed; (b) A. S. Dudnik, A. W. Scromek, M. Rubina, J. T. Kim, A. V. Kelin and V. Gevorgyan, J. Am. Chem. Soc., 2008, 130, 1440 CrossRef CAS PubMed; (c) F. Chen, T. Shen, Y. Cui and N. Jiao, Org. Lett., 2012, 14, 4926 CrossRef CAS PubMed; (d) W. Liu, H. Jiang and L. Huang, Org. Lett., 2010, 12, 312 CrossRef CAS PubMed; (e) H. Dong, M. Shen, J. E. Redford, B. J. Stokes, A. L. Pumphrey and T. G. Driver, Org. Lett., 2007, 9, 5191 CrossRef CAS PubMed; (f) B. Gabriele, G. Salerno and A. Fazio, J. Org. Chem., 2003, 68, 7853 CrossRef CAS PubMed.
- (a) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (b) M. K. Engle, T. S. Mei, M. Wasa and J. Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef PubMed; (c) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814 CrossRef CAS PubMed; (d) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651 RSC; (e) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (f) J. Wencel-Delord, T. Droge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (g) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS PubMed; (h) C. L. Sun, B. J. Li and Z. J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (i) P. Herrmann and T. Bach, Chem. Soc. Rev., 2011, 40, 2022 RSC; (j) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (k) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (l) Topics in Current Chemistry, ed. J. Q. Yu and Z. J. Shi, Springer, Berlin, 2010, vol. 292 Search PubMed; (m) A. S. Dudnik and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2096 CrossRef CAS PubMed; (n) B. Li, N. Wang, Y. Liang, S. Xu and B. Wang, Org. Lett., 2013, 15, 136 CrossRef CAS PubMed.
- (a) J. Zhu and H. Bienyame, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) I. Nakamura and Y. Yamamoto, Chem. Rev., 2004, 104, 2127 CrossRef CAS PubMed; (c) T. Vlaar, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Angew. Chem., Int. Ed., 2013, 52, 7084 CrossRef CAS PubMed; (d) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS; (e) H. Clavier and H. Pellissier, Adv. Synth. Catal., 2012, 354, 3347 CrossRef CAS; (f) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed; (g) B. Jiang, T. Rajale, W. Wever, S. J. Tu and G. Li, Chem. – Asian J., 2010, 5, 2318 CrossRef CAS PubMed; (h) L. A. Wessjohann, D. G. Rivera and O. E. Vercillo, Chem. Rev., 2009, 109, 796 CrossRef CAS PubMed; (i) B. B. Toure and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef CAS PubMed; (j) D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef CAS PubMed.
- (a) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Chem. – Eur. J., 2012, 18, 5460 CrossRef CAS PubMed; (b) D. Tejedor, D. Gonzalez-Cruz, A. Santos-Exposito, J. J. Marrero-Tellado, P. de Armas and F. Garcia-Tellado, Chem. – Eur. J., 2005, 11, 3502 CrossRef CAS PubMed; (c) D. L. Priebbenow, F. M. Pfeffer and S. G. Stewart, Eur. J. Org. Chem., 2011, 1632 CrossRef CAS; (d) M. J. Bouma, G. Masson and J. Zhu, Eur. J. Org. Chem., 2012, 475 CrossRef CAS; (e) Y. Liu and J. P. Wan, Chem. – Asian J., 2012, 7, 1488 CrossRef CAS PubMed; (f) B. Jiang, Q. Y. Li, S. J. Tu and G. Li, Org. Lett., 2012, 14, 5210 CrossRef CAS PubMed; (g) M. Presset, Y. Coquerel and Rodriguez, Org. Lett., 2009, 11, 5706 CrossRef CAS PubMed; (h) Y. Ohta, H. Chiba, S. Oishi, N. Fujii and H. Ohno, J. Org. Chem., 2009, 74, 7052 CrossRef CAS PubMed; (i) J. Sun, Y. Sun, H. Gong, Y. J. Xie and C. G. Yan, Org. Lett., 2012, 14, 5172 CrossRef CAS PubMed; (j) S. Chowdhury, G. C. Nandi, S. Samai and M. S. Singh, Org. Lett., 2011, 13, 3762 CrossRef CAS PubMed; (k) A. Moyano and R. Rios, Chem. Rev., 2011, 111, 4703 CrossRef CAS PubMed.
- B. Das, N. Bhunia and M. Lingaiah, Synthesis, 2011, 3471 CrossRef CAS PubMed.
- (a) S. P. Andrews, A. F. Stepan, H. Tanaka, S. V. Ley and M. D. Smith, Adv. Synth. Catal., 2005, 347, 647 CrossRef CAS; (b) T. Miao and L. Wang, Tetrahedron Lett., 2007, 48, 95 CrossRef CAS PubMed; (c) A. J. Amali and K. R. Rana, Green Chem., 2009, 11, 1781 RSC; (d) M. Cai, G. Zheng, L. Zha and J. Peng, Eur. J. Org. Chem., 2009, 1585 CrossRef CAS; (e) A. M. Trzeciak, E. Mieczynska, J. J. Ziolkowski, W. Bukowski, A. Bukowska, J. Noworol and J. Okal, New J. Chem., 2008, 32, 1124 RSC; (f) X. Ma, Y. Zhou, J. Zhang, A. Zhu, T. Jiang and B. Han, Green Chem., 2008, 10, 59 RSC; (g) K. Mennecke and A. Kirschning, Beilstein J. Org. Chem., 2009, 5, 3762 Search PubMed.
- J. R. Hwu, M. L. Jain, F. Y. Tsai, S. C. Tsay, A. Balakumar and G. H. Hakimelahi, J. Org. Chem., 2000, 65, 5077 CrossRef CAS.
- (a) V. Nair and A. Deepthi, Chem. Rev., 2007, 107, 1862 CrossRef CAS PubMed; (b) S. Vellaisamy and J. C. Meneendez, Org. Lett., 2008, 10, 4303 CrossRef PubMed; (c) K. T. Pradip and M. Chhanda, ARKIVOC, 2011, 10, 287 Search PubMed.
- A. B. Atar, S. D. Dindulkar and Y. T. Jeong, Monatsh. Chem., 2013, 144, 695 CrossRef CAS.
- A. B. Atar, Y. S. Jeong and Y. T. Jeong, Tetrahedron, 2014, 70, 5207 CrossRef CAS PubMed.
- A. B. Atar and Y. T. Jeong, Mol. Diversity, 2014, 18, 389 CrossRef CAS PubMed.
- A. B. Atar, J. Oh, J. T. Kim and Y. T. Jeong, Monatsh. Chem., 2014, 145, 329 CrossRef CAS PubMed.
- R. H. Jih, L. J. Moti, T. Fu-Yuan, T. Shwu-Chen, B. Arumugham and H. H. Gholam, J. Org. Chem., 2000, 65, 5077 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nj01234h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |