Novel room-temperature thermotropic liquid crystals: synthesis and mesomorphism of gallic–perylene–gallic trimers†
Li
Meng
a,
Qiumei
Wu
a,
Fafu
Yang
*ab and
Hongyu
Guo
a
aCollege of Chemistry and Chemical Engineering, Fujian Normal University, Fuzhou 350007, P. R. China. E-mail: yangfafu@fjnu.edu.cn; Tel: +86-591-83465225
bFujian Key Laboratory of Polymer Materials, Fuzhou 350007, P. R. China
First published on 12th September 2014
Abstract
Three novel gallic–perylene–gallic trimers 7, 8 and 9 were synthesized by reacting gallic amino derivatives 3, 4 or 6 with perylene tetracarboxylic anhydride in yields of 75%, 78% and 80%, respectively. Compounds 7 and 8 with soft bridging chains exhibit columnar mesophase at room temperature, but compound 9 with a rigid bridging chain exhibits no mesophase.
Discotic liquid crystals (LC)—with readily self-assembling structures to construct ordered columnar phases that can, in turn, lead to efficient, directional charge migration—are outstanding candidates for use in organic photoelectric devices, photovoltaic devices, thin film transistors, organic light emitting diodes, etc.1,2 Till date, some discotic liquid crystals, such as triphenylene-based liquid crystals3–6 and phthalocyanine-based liquid crystals,7–9 have been investigated extensively.
Perylene derivatives with aromatic imide groups are usually planar, rigid, polar and thermostable. These structural characteristics are favorable for using them as rigid components in organic discotic liquid crystal materials.10 Moreover, perylenes have attracted particular attention due to their excellent absorption and emission properties in both solution and solid state. For example, a perylene bisimide derivative exhibits outstanding photovoltaic performance in conjunction with hexabenzocoronene discotic materials.11 Furthermore, perylene diimide derivatives possess compact and electron deficient cores, demonstrating great potential as n-type (electron-transporting) semiconductors in comparison to their more common p-type counterparts in organic semiconductors.12,13 Due to these excellent photoelectric properties, all kinds of perylene derivatives, including perylene liquid crystals, have been reported to date. Generally, perylene liquid crystals were obtained by attaching multiple long aliphatic chains at the imide positions.14 The first perylene liquid crystals with polyoxyethylene chains were described by Cormier and Gregg.15 Several perylene–triphenylene derivatives were synthesized as good liquid crystal materials.16–18 Perylene bisimide discotic liquid crystals were also prepared by modifying imide groups with siloxane,19 alkyl esters,20 organosilica,21 dendritic peptides,22 and fluoro-pentenyl groups.23 It was found in these studies that the LC properties were strongly influenced by the nature of the substituents. Most of these perylene liquid crystals possess wide temperature ranges of liquid crystalline phases. They usually display mesophase beyond the room temperature.24 However, room-temperature liquid crystal is an important property to retain the self-assembling property over long periods of time after thermal processing.22,25 To date, several room-temperature perylene liquid crystals have been reported by introducing alkoxyl or alkyl groups,26,27 tri-(dodecyloxy)phenyl groups,28 phenoxy substituents,29,30 and tridodecylphenyl groups31 onto imide or perylene cores. Lately, Percec's group also synthesized several perylene bisimides containing six long alkyl chains with interesting self-repairing complex helical columns near room temperature.32–34 Inspired by the findings described above, in this paper, we designed and synthesized three novel perylene bisimides derivatives containing two gallic acid amides units. The separating procedures were simple with recrystallization and the yields were as high as 75–80%. Moreover, the mesomorphic properties of these novel gallic–perylene–gallic trimers were studied by differential scanning calorimetry (DSC), polarized optical microscopy (POM) and X-ray diffraction (XRD) analyses. The results indicated that compounds 7 and 8 with soft bridging chains exhibit columnar mesophase at room temperature. The scopes of phase transfer temperatures are as wide as 133.9 °C and 140.7 °C, respectively. However, compound 9 with a rigid bridging chain exhibits no mesophase. This paper described two unusual room-temperature perylene liquid crystals and gave a good example on how the bridging chains significantly influence the mesomorphic properties.
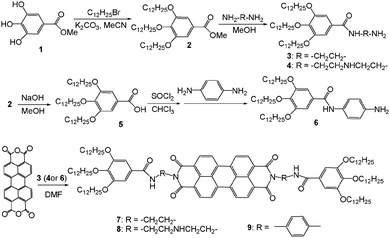
The synthetic routes of the novel gallic–perylene–gallic trimers are shown in Scheme 1. According to the literature methods,35 by reacting methyl gallate 1 with bromodecane in K2CO3–MeCN, gallic derivative 2 with three long alkyl chains was obtained in the yield of 80%. Subsequently, ammonolysis derivatives 3 and 4 were prepared by reacting compound 2 with excess ethylenediamine or diethylenetriamine in yields of 86% and 80%, respectively. Furthermore, by the hydrolysis of compound 2, subsequently converting to acyl chloride and then reacting with p-phenylene diamine, gallic derivative 6 was smoothly synthesized in the yield of 70%. Finally, by treating compound 3 (4 or 6) with perylene tetracarboxylic anhydride in dimethylformamide (DMF) at 110 °C overnight, target compounds 7, 8 and 9 were obtained in yields of 75%, 78% and 80%, respectively. The separating procedures were simple with recrystallization. It is worthy of noting that this reaction was carried out efficiently in a DMF solution without catalyst, instead of the usual reaction condition of quinoline as the solvent and zinc acetate as the catalyst.10,36
The structures of novel perylene derivatives were confirmed by the changes of the Fourier transform infrared spectroscopy spectra of perylene tetracarboxylic anhydride and compounds 7, 8 and 9. The disappearance of the anhydride C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration bands at 1770 cm−1 and the appearance of imide C
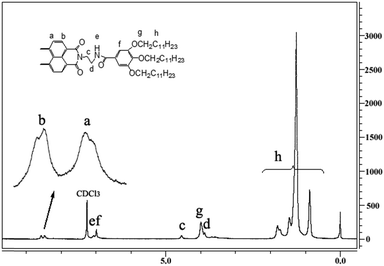
O vibration bands at 1770 cm−1 and the appearance of imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration bands at 1696 cm−1 indicate that the anhydride groups are completely substituted by amide groups. Further, the mass spectrometry (MS) spectra of compounds 7, 8 and 9 showed corresponding molecular ion peaks at 1789.4, 1875.8 and 1885.6, respectively. In the 1H nuclear magnetic resonance (NMR) spectra of compounds 7, 8 and 9, all the signals of the protons were well assigned for the structures of the target molecules. In particular, the ArH of the perylene skeleton exhibits two symmetrical doublets at 8.48 ppm and 8.60 ppm (the 1H NMR spectrum of compound 7 is illustrated in Fig. 1). These 1H NMR spectra suggested that compounds 7, 8 and 9 possess symmetrical structures that were also supported by 13C NMR. The elemental analyses were in agreement with the calculated contents of carbon, nitrogen and hydrogen in these compounds. All these characteristic data are in accordance with the structures of compounds 7, 8 and 9.
O vibration bands at 1696 cm−1 indicate that the anhydride groups are completely substituted by amide groups. Further, the mass spectrometry (MS) spectra of compounds 7, 8 and 9 showed corresponding molecular ion peaks at 1789.4, 1875.8 and 1885.6, respectively. In the 1H nuclear magnetic resonance (NMR) spectra of compounds 7, 8 and 9, all the signals of the protons were well assigned for the structures of the target molecules. In particular, the ArH of the perylene skeleton exhibits two symmetrical doublets at 8.48 ppm and 8.60 ppm (the 1H NMR spectrum of compound 7 is illustrated in Fig. 1). These 1H NMR spectra suggested that compounds 7, 8 and 9 possess symmetrical structures that were also supported by 13C NMR. The elemental analyses were in agreement with the calculated contents of carbon, nitrogen and hydrogen in these compounds. All these characteristic data are in accordance with the structures of compounds 7, 8 and 9.
The mesomorphic behaviours of compounds 7, 8 and 9 were investigated by DSC, as shown in Fig. 2. The enthalpies of the phase transitions are listed in Table 1. Their similar phase transition temperatures and enthalpies suggested that they possess good reversible phase transitions on heating and cooling. The enthalpy changes of Cr–LC were marginally smaller than that of LC–Iso, which might indicate that their crystalline phases were mixed with some amorphous phases. Compound 7 shows two phase transfer temperatures at −1.5 °C and 132.4 °C in the second heating and two reverse processes at 122.8 °C and −7.9 °C in cooling. Similarly, compound 8 exhibits two phase transfer temperatures at 15.3 °C and 135.9 °C for the second heating scan and two reverse processes at 130.3 °C and 9.3 °C upon cooling. However, compound 9 shows only one peak at second heating or cooling at 158 °C. These DSC data suggest that the crystal phase–mesophase–isotropic phase exists on the heating and cooling process for compounds 7 and 8, but no mesophase was exhibited by compound 9. Comparing with the structures of compounds 7, 8 and 9, these DSC results could be explained by that of the soft bridging chains between the gallic and perylene units in compounds 7 and 8, which are favourable for mesophase. However, the rigid bridging chain in compound 9 is an obstacle for mesophase because the core of a planar rigid π-conjugate system is too large to produce liquid crystals. Moreover, these DSC data indicate that compounds 7 and 8 exhibit the mesomorphic properties at room temperature, which were seldom observed for perylene liquid crystals.24 Further, from these DSC data, the scopes of the phase transfer temperatures are calculated to be as wide as 133.9 °C and 140.7 °C for compounds 7 and 8, respectively. These scopes of the phase transfer temperatures are wider than those of most of the reported perylene liquid crystals.24
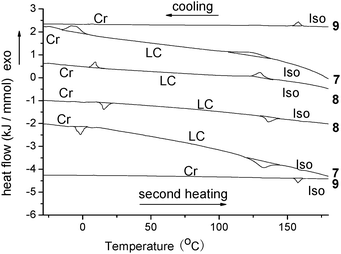 | ||
| Fig. 2 The DSC traces of compounds 7, 8 and 9 on second heating and cooling (scan rate: 10 °C min−1). | ||
Based on the DSC results, POM was used to study the mesomorphic textures of compounds 7 and 8. Fig. 3 shows their LC textures at the corresponding temperatures. It can be seen that the pseudo-confocal conic textures were observed for compounds 7 and 8. These textures are typical for columnar mesomorphism, although it is difficult to speculate the specific columnar phase (such as hexagonal or rectangular). Some researchers also reported similar results in which mesogens typically stack to form columns (not necessarily simple one-molecule-wide columns), but there is no positional order among the columns.10,19,24,36
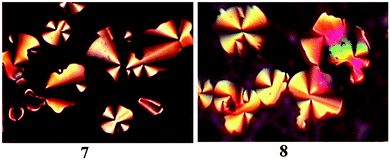 | ||
| Fig. 3 The textures of compounds 7 and 8 obtained with polarized optical microscopy on cooling at 90 °C (×400). | ||
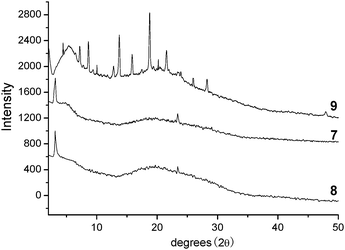
In order to further investigate the molecular stacking behaviours of the mesophase, compounds 7, 8 and 9 were investigated by XRD at corresponding temperatures (Fig. 4). In Fig. 4, the complicated peaks of compound 9 were observed for the crystalline phase. Further, the XRD of compound 9 over 158 °C showed no obvious peaks because it was at the isotropic phase. However, typical peaks of the columnar phase of perylene liquid crystals were observed for compounds 7 and 8. The reflections at about 2θ = 3.1° correspond to the distances of 28.5 Å, which are in reasonable agreement with the molecular length of the perylene diimide group. The broad halos at 2θ = 15°–25° are ascribed to the average distances of the molten alkyl chains. The reflections at 23.5° indicate the spacing of approximately 3.7 Å, which is the typical characteristic of intracolumnar order of the discotic liquid crystals with π–π interactions. Although no other obvious XRD reflection is observed to further support the columnar phase, it is reasonable to deduce the existence of columnar liquid crystals based on these XRD data and the above POM results. These deductions were also in accordance with the similar perylene bisimides containing six long alkyl chains possessing complex helical columns reported by Percec's group lately.32–34 However, it is difficult to deduce the specific phase structures of compounds 7 and 8, such as hexagonal or rectangular liquid crystal phases, due to the absence of characteristic peaks on the XRD traces. These XRD results might imply no positional order among the columns, which is in accordance with the analysis of the POM results and other columnar perylene liquid crystals with no positional order.10,19,24,36
Combining all the mesomorphic experimental results of DSC, POM and XRD data, it could be concluded that compounds 7 and 8 exhibit columnar mesophases at room temperature and possess wide scopes of phase transfer temperatures, although it is difficult to speculate the specific phase structures.
In conclusion, three novel gallic–perylene–gallic trimers were synthesized conveniently in yields of 75–80% and possess symmetrical structures. Their mesomorphic properties were studied by the DSC, POM and XRD analyses. Compounds 7 and 8 with soft bridging chains show mesophase at the room temperature with wide scopes of phase transfer temperatures (133.9 °C and 140.7 °C, respectively), but compound 9 with rigid bridging chains exhibits no mesophase. The POM and XRD results indicate that compounds 7 and 8 possess columnar mesophases. These gallic–perylene–gallic trimers are the first liquid crystals of perylene bisimides possessing amide and amine groups, and their unusual mesomorphic properties at room temperature were seldom observed for perylene derivatives. This paper also gave a good example on how the bridging chains considerably influence the mesomorphic properties. Based on these research results, other gallic–perylene–gallic trimers with various bridging chains, which are expected to have unique mesomorphic properties, are being investigated in the subsequent works.
Experimental
General
All the chemical reagents were obtained from commercial suppliers. The other organic solvents and inorganic reagents were purified according to standard anhydrous methods before use. NMR spectra were recorded in CDCl3 on a Bruker-ARX 400 instrument at 30 °C. The MS spectra were obtained from DECAX-30000 LCQ Deca XP mass spectrometer. The elemental analyses were performed using the Vario EL III Elemental Analyzer. POM (Leica DMRX) was used along with a hot stage (Linkam THMSE 600) to examine the phase transitions. The thermal analysis of the materials was carried out using DSC (Thermal Analysis Q100) at a scanning rate of 10 °C min−1 under N2 atmosphere. The XRD experiments were performed with a SEIFERT-FPM (XRD7) using Cu Kα 1.5406 Å as the radiation source with power of 40 kV and 30 mA.Synthetic procedures of compounds 7, 8 and 9
Perylene tetracarboxylic anhydride (0.196 g, 0.5 mmol) and compound 3 (4 or 6) (1 mmol) were stirred and heated in DMF (30 mL) at 110 °C overnight. TLC detection indicated the disappearance of materials. Then, distilled water was added in the reaction mixture, and the produced red precipitates were filtered. The red precipitates were washed with methanol. The crude products were purified by recrystallization with CHCl3–MeOH. The red soft solids were obtained in the yields of 75%, 78% and 80%, respectively. Compound 7: 1H NMR (400 MHz, CDCl3) δ ppm: 0.88 (bs, 18H, CH3), 1.24–1.80 (m, 120H, CH2), 3.89 (bs, 4H, CH2), 3.99 (bs, 12H, CH2), 4.55 (bs, 4H, CH2), 6.99 (s, 4H, ArH), 7.07 (s, 2H, NH), 8.48 (d, J = 7.6 Hz, 4H, ArH), 8.60 (d, J = 7.6 Hz, 4H, ArH); 13C NMR (150 MHz, CDCl3) δ ppm: 14.049, 22.628, 26.036, 26.110, 29.170, 29.311, 29.411, 29.545, 29.636, 30.281, 31.862, 39.747, 69.096, 73.401, 105.474, 122.750, 123.072, 125.797, 125.867, 129.050, 131.257, 134.220, 140.792, 152.894, 163.690, 167.557; MS m/z (%): 1789.4 (M+, 100). Anal. calcd for C114H172N4O12: C 76.47, H 9.68, N 3.13; found C 76.41, H 9.73, N 3.07%. Compound 8: 1H NMR (400 MHz, CDCl3) δ ppm: 0.89 (bs, 18H, CH3), 1.25–1.79 (m, 120H, CH2), 3.25 (bs, 2H, NH), 3.51–4.61 (m, 28H, OCH2 and NCH2), 6.98 (s, 4H, ArH), 7.08 (s, 2H, NH), 8.46 (d, J = 7.6 Hz, 4H, ArH), 8.58 (d, J = 7.6 Hz, 4H, ArH); MS m/z (%): 1875.8 (M+, 100). Anal. calcd for C118H182N6O12: C 75.52, H 9.77, N 4.48; found C 75.47, H 9.72, N 4.44%. Compound 9: 1H NMR (400 MHz, CDCl3) δ ppm: 0.88 (bs, 18H, CH3), 1.23–1.81 (m, 120H, CH2), 3.97 (bs, 12H, CH2), 6.99 (bs, 4H, ArH), 7.03 (s, 2H, NH), 8.49 (d, J = 6.8 Hz, 4H, ArH), 8.54 (s, 8H, ArH), 8.56 (d, J = 6.8 Hz, 4H, ArH); MS m/z (%): 1885.6 (M+, 100). Anal. calcd for C122H172N4O12: C 76.67, H 9.19, N 2.97; found C 76.61, H 9.25, N 3.03%.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21406036), the Fujian Natural Science Foundation of China (No. 2014J01034), the Program for Innovative Research Team in Science and Technology in Fujian Province University and the Program of students innovative experimental projects of FJNU (201310394016) are greatly acknowledged.Notes and references
- D. Adam, P. Schuhmacher, J. Simmerer, L. Häussling, K. Siemensmeyer, K. H. Etzbach, H. Ringsdorf and D. Haarer, Nature, 1994, 371, 141 CrossRef CAS.
- W. Pisula, A. Menon, M. Stepputat, I. Lieberwirth, A. Kolbe, A. Tracz, H. Sirringhaus, T. Pakula and K. Müllen, Adv. Mater., 2005, 17, 684 CrossRef CAS.
- F. F. Yang, X. Y. Bai, H. Y. Guo and C. C. Li, Tetrahedron Lett., 2013, 54, 409 CrossRef CAS PubMed.
- F. F. Yang, B. T. Xu, H. Y. Guo and J. W. Xie, Tetrahedron Lett., 2012, 53, 1598 CrossRef CAS PubMed.
- F. F. Yang, H. Y. Guo, J. W. Xie and J. R. Lin, Eur. J. Org. Chem., 2011, 5141 CrossRef CAS.
- F. F. Yang, J. W. Xie, H. Y. Guo, B. T. Xu and C. C. Li, Liq. Cryst., 2012, 39, 1368 CrossRef CAS.
- M. Ichihara, A. Suzuki, K. Hatsusaka and K. Ohta, Liq. Cryst., 2007, 34, 555 CrossRef CAS.
- C. H. Lee, Y. W. Kwon, D. H. Choi, Y. H. Geerts, E. Koh and J. I. Jin, Adv. Mater., 2010, 22, 4405 CrossRef CAS PubMed.
- S. Makhseed, A. Bumajded, B. Ghanem, K. Msayib and N. B. McKeown, Tetrahedron Lett., 2004, 45, 4865 CrossRef CAS PubMed.
- A. Wicklein, A. Lang, M. Much and M. Thelakkat, J. Am. Chem. Soc., 2009, 131, 14442 CrossRef CAS PubMed.
- L. Schmidt-Mende, A. Fechtenkötter, K. Müullen, E. Moons, R. H. Friend and J. D. MacKenzie, Science, 2001, 293, 1119 CrossRef CAS PubMed.
- C. W. Struijk, A. B. Sieval, J. E. J. Dakhorst, M. van Dijk, P. Kimkes, R. B. M. Koehorst, H. Donker, T. J. Schaafsma, S. J. Picken, A. M. van de Craats, J. M. Warman, H. Zuilhof and E. J. R. Sudhöler, J. Am. Chem. Soc., 2000, 122, 11057 CrossRef CAS.
- T. B. Singh, S. Erten, S. Gunes, C. Zafer, G. Turkmen, B. Kuban, Y. Teoman, N. S. Sariciftci and S. Icli, Org. Electron., 2006, 7, 480–489 CrossRef CAS PubMed.
- M. M. Safont-Sempere, V. Stepanenko, M. Lehmann and F. Wurthner, J. Mater. Chem., 2011, 21, 7201 RSC.
- R. A. Cormier and B. A. Gregg, Chem. Mater., 1998, 10, 1309 CrossRef CAS.
- M. Bagui, T. Dutta, H. Z. Zhong, S. H. Li, S. Chakraborty, A. Keightley and Z. H. Peng, Tetrahedron, 2012, 68, 2806 CrossRef CAS PubMed.
- M. Bagui, S. Chakraborty, J. S. Melinger, H. Z. Zhong, A. Keightley and Z. H. Peng, J. Phys. Chem. A, 2011, 115, 1579 CrossRef CAS PubMed.
- X. F. Kong, Z. Q. He, Y. N. Zhang, L. P. Mu, C. J. Liang, B. Chen, X. P. Jing and A. N. Cammidge, Org. Lett., 2011, 13, 764 CrossRef CAS PubMed.
- T. J. Zhang, D. M. Sun, X. K. Ren, L. L. Liu, G. Y. Wen, Z. J. Ren, H. H. Li and S. K. Yan, Soft Matter, 2013, 9, 10739 RSC.
- L. Wang, Q. Cui, X. F. Chen, Y. Li, Z. Q. Li, D. Wang and H. Yang, Aust. J. Chem., 2013, 66, 692 CrossRef CAS.
- N. Mizoshita, T. Tani and S. Inagaki, Adv. Funct. Mater., 2011, 21, 3291 CrossRef CAS.
- B. X. Gao, D. F. Xia, L. C. Zhang, Q. Q. Bai, L. B. Bai, T. Yang and X. W. Ba, J. Mater. Chem., 2011, 21, 15975 RSC.
- E. Wolarz, E. Mykowska, T. Martynski and R. Stolarski, J. Mol. Struct., 2009, 929, 79 CrossRef CAS PubMed.
- K. Bijak, H. Janeczek, M. Grucela-Zajac and E. Schab-Balcerzak, Opt. Mater., 2013, 35, 1042 CrossRef CAS PubMed.
- G. A. Bhavsar and S. K. Asha, Chem. – Eur. J., 2011, 17, 12646 CrossRef CAS PubMed.
- R. A. Cormier and B. A. Gregg, J. Phys. Chem. B, 1997, 101, 11004 CrossRef CAS.
- Y. Xu, S. Leng, C. Xue, R. Sun, J. Pan, J. Ford and S. Jin, Angew. Chem., Int. Ed., 2007, 46, 3896 CrossRef CAS PubMed.
- F. Würthner, C. Thalacker, S. Diele and C. Tschierske, Chem. – Eur. J., 2001, 7, 2245 CrossRef.
- Z. An, J. Yu, S. C. Jones, S. Barlow, S. Yoo, B. Domercq, P. Prins, L. D. A. Siebbeles, B. Kippelen and S. R. Marder, Adv. Mater., 2005, 17, 2580 CrossRef CAS.
- Z. Chen, U. Baumeister, C. Tschierske and F. Würthner, Chem. – Eur. J., 2007, 13, 450 CrossRef CAS PubMed.
- M. Funahashi and A. Sonoda, Org. Electron., 2012, 13, 1633 CrossRef CAS PubMed.
- V. Percec, H. Sun, P. Leowanawat, M. Peterca, R. Graf, H. Spiess, X. Zeng, G. Ungar and P. Heiney, J. Am. Chem. Soc., 2013, 135, 4129 CrossRef CAS PubMed.
- V. Percec, S. D. Hudson, M. Peterca, P. Leowanawat, E. Aqad, R. Graf, H. Spiess, X. Zeng, G. Ungar and P. Heiney, J. Am. Chem. Soc., 2011, 133, 18479 CrossRef CAS PubMed.
- V. Percec, M. Peterca, T. Tadjiev, X. Zeng, G. Ungar, P. Leowanawat, E. Aqad, M. R. Imam, B. M. Rosen, U. Akbey, R. Graf, S. Sekharan, D. Sebastiani, H. W. Spiess, P. A. Heiney and S. D. Hudson, J. Am. Chem. Soc., 2011, 133, 12197 CrossRef CAS PubMed.
- V. S. K. Balagurusamy, G. Ungar, V. Percec and G. Johansson, J. Am. Chem. Soc., 1997, 119, 1539 CrossRef CAS.
- S. Laschat, A. Baro, N. Steinke, F. Giesselmann, C. Hägele, G. Scalia, R. Judele, E. Kapatsina, S. Sauer, A. Schreivogel and M. Tosoni, Angew. Chem., Int. Ed., 2007, 46, 4832 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental procedures in details were presented in ESI. See DOI: 10.1039/c4nj00993b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |